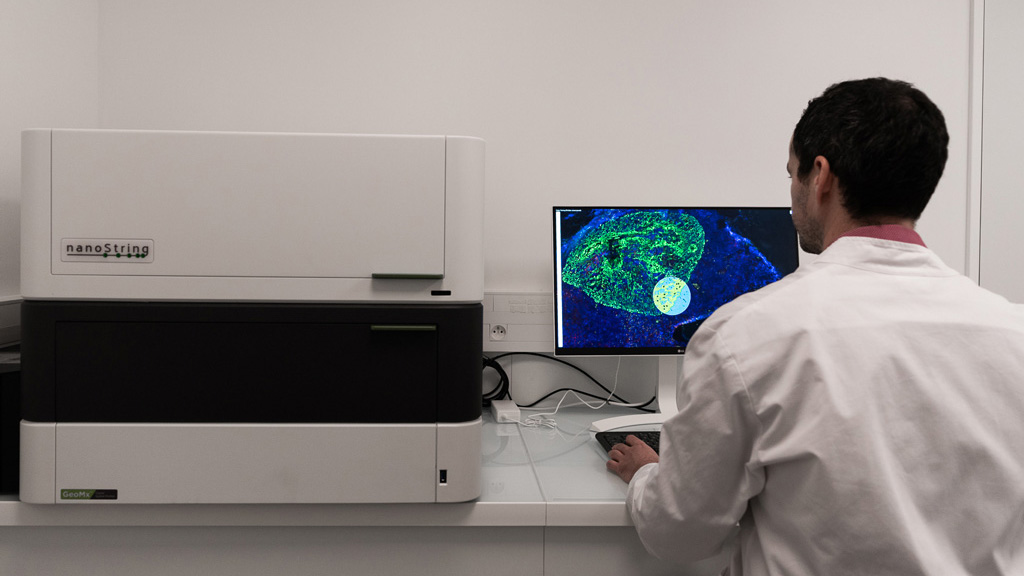
Discover our latest study, titled Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies.
Discover our latest article, published in Frontiers in Immunology.
The CONDOR consortium, led by Pr. Antoine Italiano of the Bergonié Institute (Bordeaux, France) is one of the 17 national awardees in the fifth call for projects in Hospital-University Research in Health (RHU5).
Here is our latest publication, titled Regorafenib-avelumab combination in patients with biliary tract cancer (REGOMUNE): a single-arm, open-label, phase II trial.
Here is our latest publication, titled Trabectedin plus Durvalumab in Patients with Advanced Pretreated Soft Tissue Sarcoma and Ovarian Carcinoma (TRAMUNE).
Discover why there is a crucial need to decipher mechanisms underlying sensitivity / resistance to cancer immunotherapies which can ultimately lead to the identification of novel therapeutic targets and how to achieve it.
Our data illustrated here through DC differentiation and mixed leukocyte reaction assays show that adenosine-mediated suppression results in the generation of phenotypically and functionally dysregulated DCs, and that this process is relieved by adenosine receptor antagonists.
Discover our latest publication, titled CD95/Fas protects triple negative breast cancer from anti-tumor activity of NK cells.
We have fully set up and characterized a syngeneic Lewis LLC1 mouse model for preclinical studies of novel therapeutic agents and anti-cancer strategies.
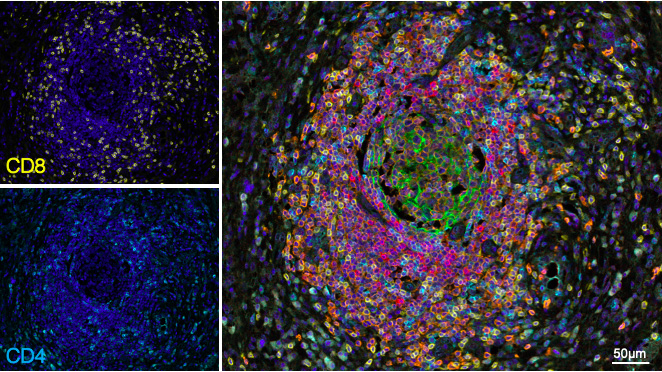
In a recent collaborative work (1) supervised by Pr A. Italiano and published in Nature Cancer (2), we highlighted that the presence of mature tertiary lymphoid structures (mTLS) – a structure of prominent B cell follicles and follicular dendritic cells adjoined to a CD4/CD8 T cell-containing zone - is a new predictive biomarker for response for immune checkpoint blockers.
Read our latest publication, titled Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1.
Read our latest publication, titled Plasma proteomics identifies leukemia inhibitory factor (LIF) as a novel predictive biomarker of immune-checkpoint blockade resistance