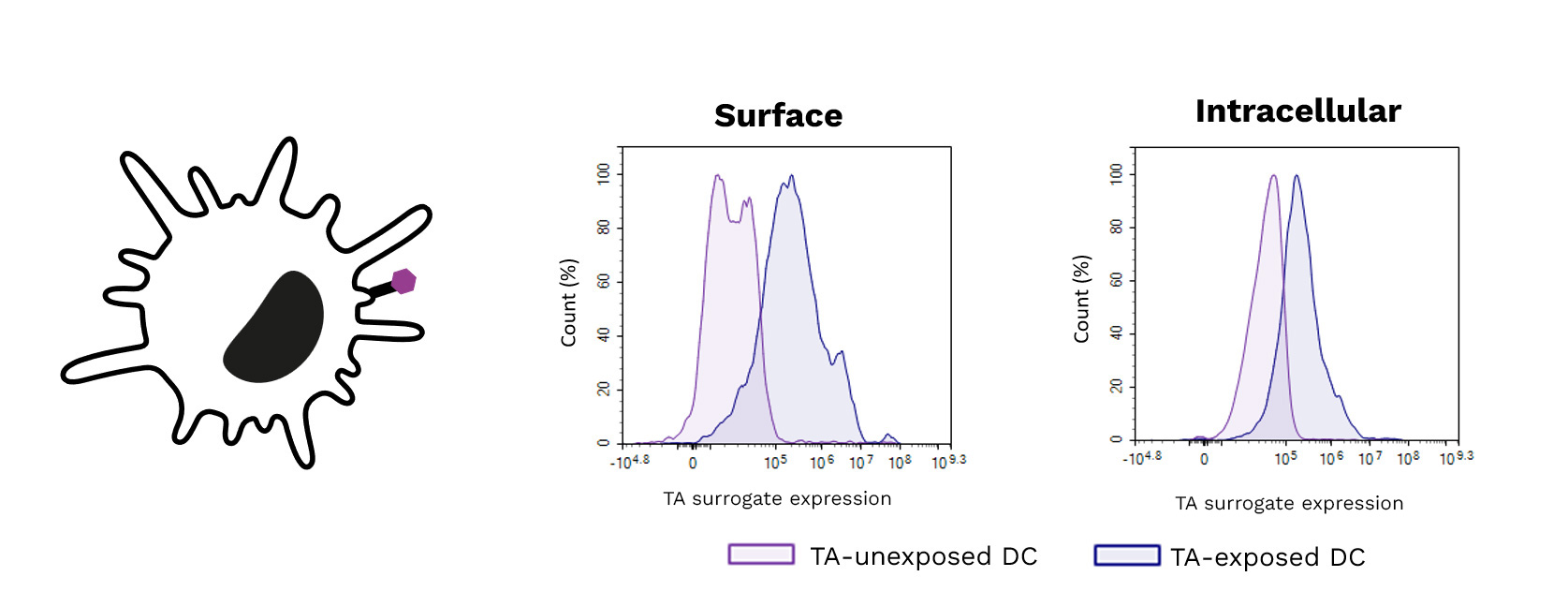
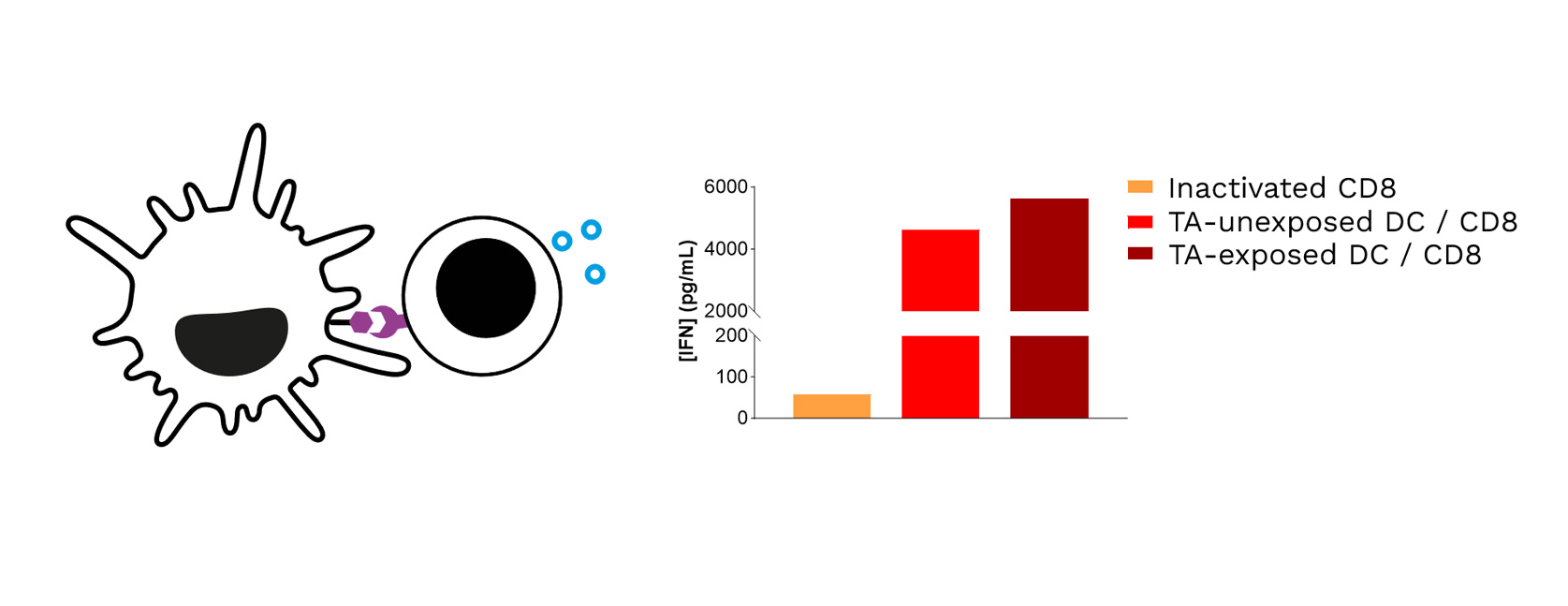
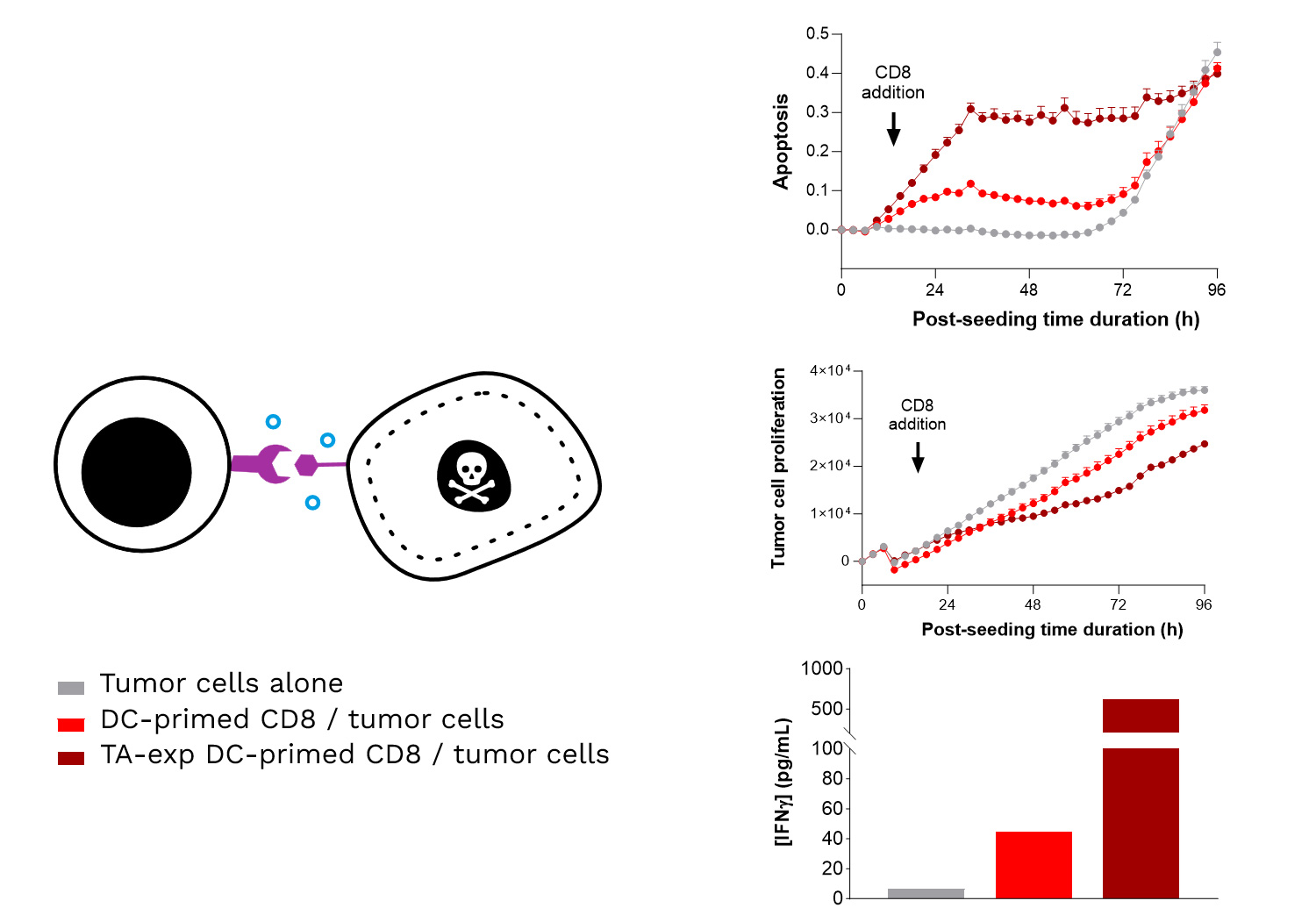
Soft-tissue sarcomas (STS) usually resist immune-checkpoint blockade because their micro-environment is both poorly immunogenic and actively immunosuppressive. To convert these “cold” into “hotter,” immune-responsive tumors, the phase II REGOMMUNE trial —led by Prof. Antoine Italiano at Institut Bergonié and Gustave Roussy—combined the multi-kinase anti-angiogenic agent regorafenib with the anti-PD-L1 antibody avelumab.
Between 2019 and 2021, 50 patients with advanced STS lacking mature tertiary lymphoid structures (TLS) were enrolled to test whether this strategy could boost immune-cell infiltration and improve anti-tumour activity.
Associated to this study through the RHU CONDOR, Explicyte profiled paired plasma samples (baseline → Cycle 2 Day 1) from 32 STS patients receiving regorafenib + avelumab with the Olink Explore HT panel (5300 proteins):
Explicyte ran multiplex immunofluorescence on paired biopsies from seven STS patients (pre- vs. Cycle 2 Day 1), corroborating the plasma data but also exposing barriers to efficacy:
Read the full paper
Between 2019 and 2021, 50 patients with advanced STS lacking mature tertiary lymphoid structures (TLS) were enrolled to test whether this strategy could boost immune-cell infiltration and improve anti-tumour activity.
Key findings published in Signal Transduction & Targeted Therapy
Modest efficacy, manageable safety
- Efficacy: Of the 43 TLS-negative patients who were assessable, the objective response rate was 11.4 % (all partial responses), whereas 50 % progressed on treatment. Median progression-free survival was 1.8 months.
- Safety: Forty-nine patients received at least one dose of either regorafenib or avelumab and were included in the safety analysis. Toxicities were generally low-grade and controllable; importantly, no treatment-related deaths occurred, indicating that the combination is well tolerated.
Plasma proteomics: stronger immune signals but red flags for checkpoint resistance
Associated to this study through the RHU CONDOR, Explicyte profiled paired plasma samples (baseline → Cycle 2 Day 1) from 32 STS patients receiving regorafenib + avelumab with the Olink Explore HT panel (5300 proteins):
- Immune-cell influx: Gene-ontology analysis of up-regulated proteins showed marked enrichment of CD8⁺ T-cell and B-cell signatures.
- Soluble PD-L1 surge: Soluble PD-L1 (CD274) —a known negative predictor of response to PD-(L)1 blockade—was the most strongly up-regulated protein.
- Tryptophan depletion: complementary metabolomics showed a pronounced drop in circulating L-tryptophan, a metabolic hallmark of immune activation.
Digital pathology: deeper—yet still not optimal—immune engagement
Explicyte ran multiplex immunofluorescence on paired biopsies from seven STS patients (pre- vs. Cycle 2 Day 1), corroborating the plasma data but also exposing barriers to efficacy:
- More lymphocytes: Intratumoral CD8⁺ T-cell and CD20⁺ B-cell densities rose in respectively 6 and 4 patients, yet progressive disease occurred in patients with the highest increases in CD8+ T and B cell density, showing that infiltration alone is insufficient to trigger clinical benefit.
- No TLS formation: Lymphocytes failed to organise into productive immune niches.
- Immunosuppressive myeloid niche: The density of M2-polarised macrophages increased, likely damping cytotoxic activity.
- Adaptive PD-1 up-shift: Post-treatment T cells displayed higher PD-1 expression, suggesting re-engagement of the PD-1/PD-L1 axis and a mechanism of resistance to avelumab.
Read the full paper