At your side
to pioneer
precision oncology
therapies

We are a specialty contract research organization offering research services in precision oncology & immuno-oncology.
Based in France, we have assisted international biotech, AI, and pharma companies, as well as comprehensive cancer centers for the past 10 years.
Our expertise in cancer immunotherapies
Preclinical services
Assessing the efficacy, MoA & safety profile of drug candidates in advanced cellular models.
NEWS
As a first biomarker assay developed under this quality management system, Explicyte now offers a multiplex immunofluorescence (IF) test to detect and score tertiary lymphoid structures (TLS) in tumor samples, supporting the identification of patients most likely to benefit from cancer immunotherapies.
https://youtu.be/bmokMO6jCHc
A 10-year track record in histopathology for immuno-oncology programs
Building on more than ten years of experience in digital pathology and translational immuno-oncology programs, Explicyte has validated 150+ tumor and immune markers across sponsored studies. The company integrates in-house FFPE tissue processing, automated IHC and IF staining, high-throughput whole-slide imaging, and standardized scoring workflows (AI- and/or pathologist-assisted) to deliver biomarker assays with high reproducibility across cohorts.
Enabling clinical-grade biomarker programs & future companion diagnostics
“Our move into clinical biomarkers is being driven by the rapid expansion of precision oncology. Drug developers increasingly rely on predictive tissue biomarkers to guide patient selection and trial stratification,” said Alban Bessède, PhD, founder and CEO of Explicyte. “While we already had the technological platforms and scientific expertise, ISO certification was essential to support sponsors at clinical stages. ISO 13485 also opens up new perspectives for the development of companion diagnostics for oncology programs.”
First validated clinical biomarker panel: multiplex TLS assessment in tumor tissues
As part of its ISO-certified tissue biomarker offering, Explicyte has developed a multiplex IF panel enabling standardized detection and scoring of TLS in tumor samples. Over the past five years, Explicyte has contributed to several translational studies published in journals such as Nature Cancer and Nature Medicine, which established the clinical relevance of TLS presence and maturity as a predictive biomarker of response to cancer immunotherapies. This validated assay, interpreted by trained pathologists, is now available for routine TLS screening in oncology clinical trials.
Biomarker services to be extended toward single-cell spatial biology
In addition to histopathology, Explicyte continues to invest in advanced translational technologies. In 2025, the company expanded its capabilities with the 10x Genomics suite of single-cell and spatial biology technologies, for which it obtained 10x Genomics’ Certified Service Provider designation.
“Our goal over the next year is to expand our ISO-certified biomarker framework to include single-cell and spatial transcriptomics workflows, as clinical programs increasingly move toward multi-omic, tissue-resolved readouts,” added Dr. Bessède.
About Explicyte's ISO-certified clinical biomarker services
Explicyte is certified ISO 13485:2016 and ISO 9001:2015 by Euro-Quality System (certificates 260133/1637F/1 and 260133/1637F/2) to support precision oncology trials with:
• Design and development of novel tissue biomarkers (single-plex and multiplex IHC/IF panels)
• Tissue biomarker testing services to support therapeutic decision-making
About Explicyte
Explicyte is a contract research organization specializing in precision oncology. Founded in 2015 by immunologist Dr. Alban Bessède, the company has supported over 100 biotech and pharmaceutical partners in the discovery and development of novel therapies for solid tumors. Based at the Institut Bergonié Comprehensive Cancer Center in Bordeaux, Explicyte brings together a multidisciplinary team of 25 scientists, including cell biologists, digital pathologists, medical oncologists, and data scientists. Over the past five years, Explicyte has co-authored 30+ peer-reviewed publications on the molecular mechanisms of response to cancer immunotherapies.
For more information, visit www.explicyte.com
Press contact: Explicyte – Pierre-Emmanuel GAULTIER - pe.gaultier@explicyte.com
🔬 What’s inside?
✅ 43 preclinical & clinical programs targeting CLDN1, CLDN4, CLDN6, or CLDN18.2
✅ Multiple modalities: mAbs (8), bispecifics (14), antibody-drug conjugates (15), CAR T cells (5)
✅ Across clinical stages: Phase I (17), Phase II (4), Phase III (10)
Featuring anti-claudin programs from leading players, including:
Alentis Therapeutics, ABL Bio, Amgen, AskGene Pharma, Astellas, AstraZeneca, BeiGene, Beijing Mabworks Biotech, BeOne Medicines, BioNTech, Bristol Myers Squibb, CARsgen Therapeutics, Chugai Pharmaceutical, CSPC ZhongQi Pharmaceutical Technology, CTTQ, Essen Biotech, Evopoint Biosciences, FutureGen Biopharmaceutical, I-Mab Biopharma, Innovent Biologics, Jiangsu Hengrui, Keymed Biosciences, LaNova Medicines, Legend Biotech, Qilu Pharmaceutical, QureBio, RemeGen, SOTIO, Suzhou Immunofoco Biotechnology, Third Arc Bio, TORL Biotherapeutics, Transcenta Therapeutics, Xencor, and Zai Lab.
Looking for a partner to advance your IO program?
Explicyte supports biotech, pharma, and AI players at every stage:
- Target discovery: We source human tumor specimens to identify and validate new therapeutic targets, leveraging multi-omic and spatial biology approaches.
- Preclinical development: With 10 years of experience assessing novel IO candidates—from hit-to-lead to pre-IND studies —we use advanced cellular models to document the efficacy, MoA and safety profile of your lead compound
- Clinical trials: ISO-certified for the development and testing of IHC/IHF-based biomarkers in clinical samples, we support patient selection and can also monitor early pharmacodynamic signals in peripheral samples.
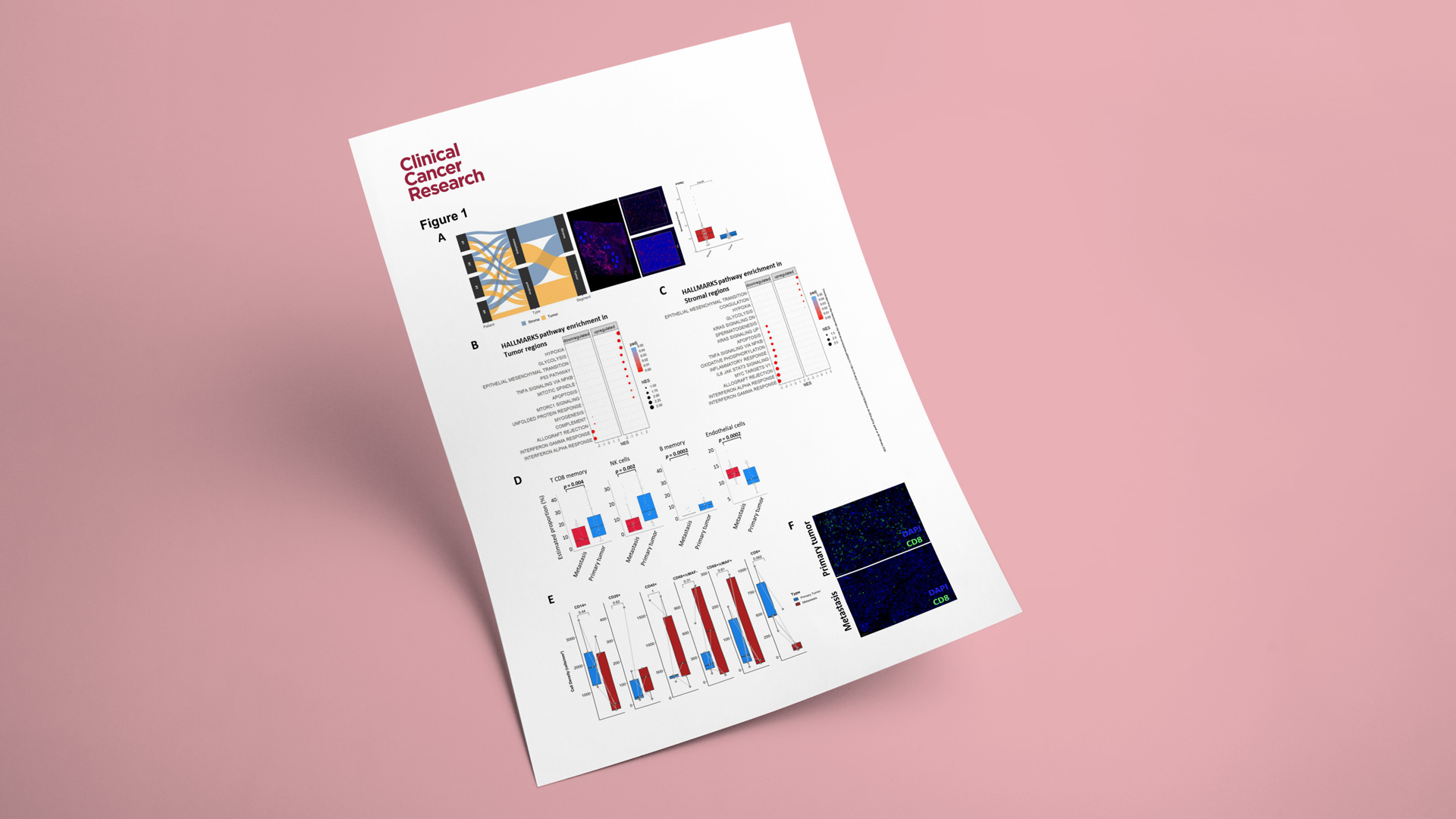
To better understand the mechanisms underlying UPS metastasis, a team led by Prof. Antoine Italiano (Institut Bergonié, University of Bordeaux, INSERM U1312 BRIC) performed an integrated multi-omics analysis of paired primary and metastatic UPS samples, followed by functional validation in relevant patient-derived models.
Spatial biology (Explicyte)
As part of this work, Explicyte performed spatial transcriptomic profiling using the GeoMx Digital Spatial Profiler (Whole Transcriptome Atlas; >18K genes). The spatial analyses were conducted on 3 paired primary tumors and matched metastases enabling a direct comparison of tumor and microenvironmental programs across disease stages.
Key observations included:
- Upregulation of hypoxia, glycolysis, and EMT-related programs in metastases, consistent with a more aggressive, metastasis-associated biology.
- Enrichment of endothelial-cell signatures in metastatic samples, suggesting increased angiogenesis compared with primary tumors.
- Higher immune infiltration in primary tumors (including CD8+ T cells, NK cells, and memory B cells), supporting the notion of a more immune-suppressive / immune-excluded state in metastatic lesions.
To further support these findings, we confirmed a reduction in CD8+ T-cell infiltration in metastases using a multiplex immunohistofluorescence (mIHF) panel in five paired primary/metastatic cases.
Bulk RNAseq
In parallel, the team analyzed paired primary and metastatic tumor samples from 13 patients using bulk RNA-seq to capture global transcriptomic changes associated with metastatic progression. This analysis confirmed an enrichment of metastasis-associated pathways and identified 690 genes significantly altered between primary tumors and metastases. Among the top candidates, ADORA2B emerged as particularly compelling because:
- ADORA2B encodes a G protein–coupled adenosine receptor (A2B) implicated in metastatic progression in several epithelial cancers,
- ADORA2B is druggable, with inhibitors already being evaluated in clinical development programs.
Using public and third-party sarcoma datasets, the team further showed that ADORA2B is overexpressed in UPS and is associated with features of an immune-suppressive microenvironment in metastatic UPS—supporting ADORA2B as a promising therapeutic target in this indication.
Functional validation
To test whether ADORA2B plays a causal role in UPS aggressiveness, the authors generated ADORA2B knockout models using CRISPR-Cas9 in two patient-derived UPS cell lines.
- Transcriptomic profiling of ADORA2B-knockout cells confirmed downregulation of key pathways involved in metastatic biology.
- Functional assays demonstrated that ADORA2B loss reduces UPS cell proliferation, migration, and invasion, supporting a direct role in tumor aggressiveness.
The in vivo impact was then evaluated in Rag2-/- γc-/- mice:
- In an orthotopic model, tumors derived from ADORA2B-knockout cells showed markedly impaired growth, leading to significantly smaller, low-proliferating tumors compared with controls.
- In a forced metastasis model (tail-vein injection), ADORA2B-knockout cells produced substantially fewer metastases, translating into a striking survival benefit (100% survival in the knockout arm vs 0% in controls in this experimental setting).
Finally, the team evaluated a dual ADORA2A/ADORA2B inhibitor, M1069 (EMD Serono), currently assessed in early-phase trials. In vitro, M1069 reduced proliferation and invasion in UPS models, providing pharmacological support for the genetic findings.
Impact
Overall, this work identifies ADORA2B as a critical regulator of primary tumor growth and metastatic dissemination in UPS. It also highlights the therapeutic promise of targeting the adenosine axis, and specifically ADORA2B, as a strategy to disrupt metastatic progression and improve outcomes in this rare and aggressive cancer.
Read the paper
This research received funding from the Agence Nationale de la Recherche (“France 2030” / ANR 21 RHUS 0010).
Using single-cell and spatial transcriptomics profiling of vitiligo patient biopsies, the team demonstrates that similar CD8+ T-cell clusters infiltrate both lesional and non-lesional skin. The difference lies in the “regulatory layer”: normal-appearing skin is characterized by an enrichment in immune regulatory pathways (increased Treg infiltration and higher PD-1 expression on CD8+ T cells), consistent with tighter regulation of inflammation.
Building on the Phase 2 BARVIT trial (NCT04822584), which showed a clinical benefit of baricitinib combined with phototherapy for repigmentation, ImmunoConcEpT partnered with Explicyte to perform a paired pre/post immune profiling of skin biopsies using our automated multiplex IF workflow.
Our multiplex IF data support the proposed mechanism; after 9 months of treatment, non-lesional skin exhibits:
• reduced CD8+ T-cell infiltration
• increased PD-1 on CD8+ T cells and PD-L1 on dendritic cells
• an increased FOXP3+ / CD8+ T-cell ratio
Together, these results highlight the critical role of immune regulatory mechanisms to prevent inflammation & depigmentation in vitiligo.
> Read the article
Explicyte acquired its first Xenium platform in October 2024 to accelerate the discovery of novel targets and biomarkers, and support drug development programmes in oncology through single-cell spatial transcriptomics.
Within a year, the company profiled more than 150 tissue specimens with Xenium, doubled its data science team, announced a partnership with Cure51 for the analysis of tumor samples from exceptional cancer survivors, and developed new methods to use Xenium in the context of cell-based assays.
“Despite optimizing the number of samples per run, we still reached our maximum capacity very quickly over 2025. To be able to offer additional slots and ensure fast turnaround times, we needed a second Xenium » said Jean-Philippe Guégan, PhD, Chief Technology Officer at Explicyte. « Our sponsors in the pharma, biotech and AI industry can’t delay their programmes — they look for availability, throughput, and robust data. With this new infrastructure, our message is clear: if you need Xenium data fast and in line with industry standards, Explicyte is your partner of choice.”
https://youtu.be/zBbs6uRHXxg
About Explicyte
Explicyte is a preclinical and translational contract research organization specializing in precision oncology. Founded in 2015 by immunologist Dr. Alban Bessède, the company has supported over 100 biotech and pharmaceutical partners in the discovery and development of novel therapies for solid tumors. Based at the Institut Bergonié Comprehensive Cancer Center in Bordeaux, Explicyte brings together a multidisciplinary team of 25 scientists, including cell biologists, digital pathologists, medical oncologists, and data scientists. Over the past five years, Explicyte has co-authored 30+ peer-reviewed publications on the molecular mechanisms of response to cancer immunotherapies.
For more information, visit www.explicyte.com
Press contacts
Explicyte – Pierre-Emmanuel GAULTIER - pe.gaultier@explicyte.com - +33 6 450 600 49
Our 2025 database of anti-DLL3 therapeutic programmes in oncology is available for free download here.
🔬 What’s inside?
✅ 6 DLL3-targeted antibody–drug conjugate (ADC) programmes
✅ 7 bispecific and trispecific antibodies
✅ 4 CAR-T & CAR-NK programmes
✅ 1 preclinical DLL3-targeting radiotherapy (RT) programme
❌ 4 discontinued anti-DLL3 clinical trials
Featuring anti-DLL3 programmes addressing solid tumours from leading players, including:
AbbVie, Amgen, Biocytogen, Boehringer Ingelheim, Chugai Pharmaceutical, CStone Pharma, Dragonfly Therapeutics, Harpoon Therapeutics, Hengrui, IDEAYA, Legend Biotech, MediLink Therapeutics, Merck, Molecular Partners, Novartis, Orano Med, Qilu Pharmaceuticals, Roche, Shanghai Fudan-Zhangjiang BioPharma, Suzhou Suncadia Biopharmaceuticals, Stemcentrx, Zai Lab, Zymeworks, and more!
Bordeaux, France — November 4, 2025. Explicyte, a French precision oncology contract research organization (CRO), today announced it has received 10x Genomics’ Certified Service Provider designation across Xenium, Visium HD and Chromium X—making Explicyte the first service provider in France certified to run single-cell spatial transcriptomic studies with Xenium. Since 2019, Explicyte has pioneered the applications of spatial transcriptomics in oncology, to discover new drug targets and biomarkers, contributing to 30+ publications in leading journals, including Nature Medicine, Nature Cancer, Molecular Cancer, and Clinical Cancer Research.
https://youtu.be/zBbs6uRHXxg
A platform built for industry needs
In 2024–2025, Explicyte unified 10x Genomics’ single-cell and spatial biology platforms into an integrated service offering for academic, pharmaceutical, biotechnology, and AI partners in precision oncology.
“An industry-grade spatial biology platform in oncology requires three major things: capacity to meet sponsors’ timelines, quality from samples to final data, and expertise to extract maximum value from precious samples,” said Alban Bessède, PhD, co-founder and CEO of Explicyte. “With that in mind, we integrated 10x technologies into our workflows.
We design studies around each specific sponsor’s scientific question—optimizing multiplexing, platform selection, and panel choices to meet time and budget constraints. We’ve established partnerships with accredited biobanks and pathologists to source and qualify unique human specimens with clinical metadata. Our quality system is aligned to ISO 13485, with certification targeted in 2026. And we’ve reinforced our data-science team and IT infrastructure to process large datasets efficiently. Within this platform, Xenium plays a central role, providing unique insights in both translational and preclinical studies.”
Scaling Xenium in tissue—and extending to in vitro
In 2025, Explicyte analyzed 100+ tissue specimens on Xenium, delivering single-cell spatial insights to uncover new targets, characterize mechanisms of action, and identify predictive and prognostic biomarkers. As a recent example, Explicyte announced a partnership with Cure51, leveraging Xenium to analyze tumor samples from exceptional cancer survivors across 50+ countries.
Beyond tissue applications, Xenium also supports in vitro research. In September 2025, Explicyte released a case study demonstrating precise immunophenotyping alongside gene-expression readouts in a multiplexed, rapid, and cost-efficient Xenium workflow.
“Single-cell resolution opens new vistas on disease biology and drug mechanisms,” added Alban Bessède. “As the first certified Xenium provider within the immuno-oncology CRO space, our mission is to equip biopharma frontrunners with data they can trust - data that de-risk and accelerate their therapeutic pipelines.»
About Explicyte
Explicyte is a preclinical and translational contract research organization specializing in precision oncology. Founded in 2015 by immunologist Dr. Alban Bessède, the company has supported over 100 biotech and pharmaceutical partners in the discovery and development of novel therapies for solid tumors. Based at the Institut Bergonié Comprehensive Cancer Center in Bordeaux, Explicyte brings together a multidisciplinary team of 25 scientists, including cell biologists, digital pathologists, medical oncologists, and data scientists. Over the past five years, Explicyte has co-authored 30+ peer-reviewed publications on the molecular mechanisms of response to cancer immunotherapies.
For more information, visit www.explicyte.com
Press contact
Pierre-Emmanuel GAULTIER - pe.gaultier@explicyte.com - +33 6 450 600 49
Targeting Tregs in Solid Tumors: Anti-CCR8 Therapeutics & Translational Insights (45-min webinar)
October 28, 2025 I 4 PM CET I 11 AM EDT
Anti-CCR8 Antibodies: From Treg Depletion to Immune Reawakening
Stephan Schann, PhD – CSO, Domain Therapeutics
Dr. Schann will present Domain’s novel differentiated strategy for selective Treg depletion and introduce DT-7012, its clinical Treg-depleting anti-CCR8 antibody candidate. He will outline the clinical rationale for targeting CCR8 in solid tumors, highlight DT-7012’s differentiation from other anti-CCR8 antibodies currently in the clinic, and show how this best-in-class candidate is engineered to overcome immune resistance and deliver durable responses—even in CCL1-rich tumors and anti-PD-1-refractory settings.
CCR8⁺ Tregs and Their Correlation with Immunotherapy Response in Advanced NSCLC
Alban Bessède, PhD – CEO, Explicyte
Dr. Bessède will present a collaborative study between Explicyte, Institut Bergonié, Gustave Roussy, and Bayer, analyzing an NSCLC cohort (BIP, NCT02534649) treated with standard-of-care immune checkpoint inhibitors. Using a validated 6-plex IHF panel, CCR8⁺ Tregs were quantified in pretreatment tumor samples and correlated with clinical outcomes (PFS, ORR), immune contexture (inflamed/infiltrated, excluded, or desert), PD-L1 TPS, and TLS status. The analysis highlights the differential predictive impact of CCR8⁺ Tregs in NSCLC, with a specific negative influence in TLS-positive tumors.
👉 Register now
📥 Bonus: Download our free landscape of anti-CCR8 therapeutics in development
Explicte: Precision Oncology & Immuno-Oncology CRO – From Target Discovery to Clinical Insight
Explicyte immuno-oncology offers preclinical contract research services for cancer immunotherapy drug discovery and translational research services. Our preclinical CRO services include the cell-based assays – for the phenotypic screening of novel immunotherapeutics and the in vitro determination of the molecular mechanism of action of new anti-cancer immune modulators. In addition, we provide translational services in immuno-oncology. Working with patient biopsies (liquid or solid samples), we can assist clinicians in the discovery of novel cancer targets and biomarkers of cancer immunotherapies. In the context of clinical trials, we offer biomarker testing and biomarker research services. Our flow cytometry team has a 10-year expertise in biomarker analysis in plasma, serum, and PBMC samples. We also offer to analyze tumor biopsies using our spatial biology platform, combining digital pathology, single-cell spatial transcriptomics (Xenium), and digital spatial profiling (spatial transcriptomics using the Nanostring GeoMx ). Our Omics capacities also include single-cell RNA sequencing (10x Chromium) and Olink-based proteomics. All our drug development services for cancer immunotherapy are performed in Bordeaux, France, serving clients all around the world, including sponsors in Europe (EU and outside), Germany, Belgium, Spain, UK, USA, Canada, Japan, and Korea.

















![[webinar] Targeting Tregs in Solid Tumors: Anti-CCR8 Therapeutics & Translational Insights Targeting Tregs in Solid Tumors Anti-CCR8 Therapeutics & Translational Insights](https://explicyte.com/wp-content/uploads/2025/10/Targeting-Tregs-in-Solid-Tumors-Anti-CCR8-Therapeutics-Translational-Insights.jpg)