Explicyte’s expertise and advanced technologies have been harnessed so as to build a cell-based service platform for immuno-oncology (IO) assessment and drug discovery.
Being sensitive, quantitative, and high throughput-compatible, our assays are not solely based on the analysis of phenotypic parameters to assess the immune response, but also consist in measuring an array of cytokines – pro- or anti-inflammatory – directly underlying the specific response of every immune cell subsets. Measurements of these cytokines in our various in vitro IO cell-based assays constitute relevant surrogates to evaluate therapeutics for their modulatory potential of immune cell functions.
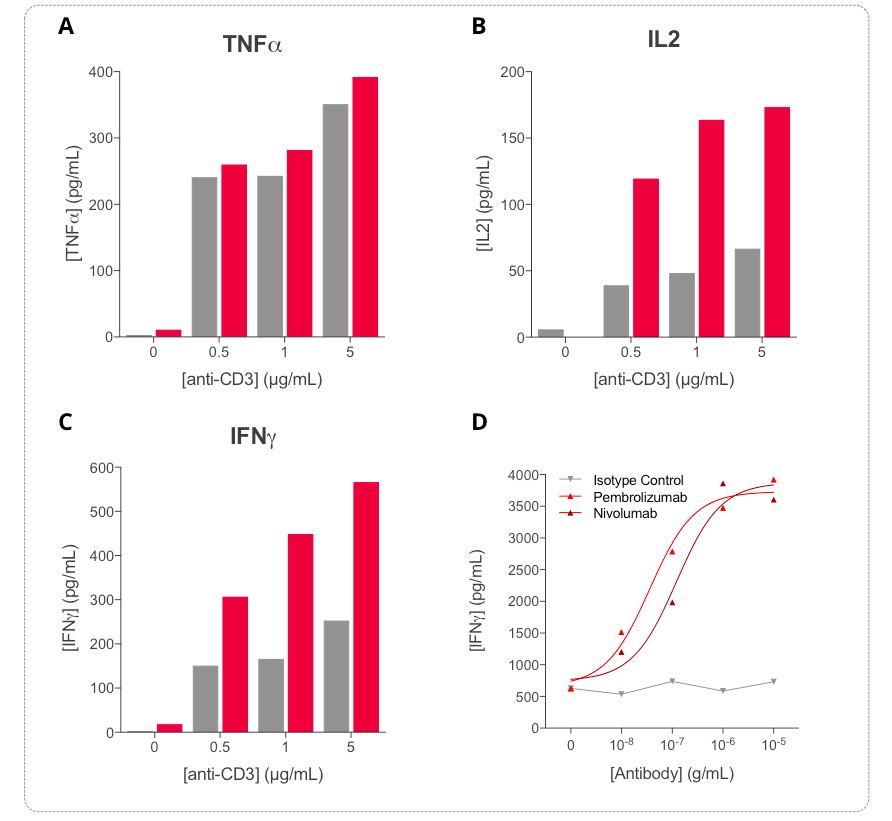
1. PBMC release of key inflammatory cytokines is induced upon CD3 activation and further optimized by immune checkpoint inhibitors
Key inflammatory cytokines i.e. TNF𝞪 (A), IL2 (B), and IFN𝞬 (C) are shown to be differentially modulated upon stimulation of PBMCs with increasing concentrations of anti-CD3 antibody. While TNFa secretion is efficiently induced at 24h (gray bars) compared to 72h (red bars), released IFNg and IL2 levels are time- and dose-dependently increased following anti-CD3 treatment (24h in gray and 72h in red). Cytokine quantification, especially IL2 and IFNg quantified by HTRF, appears valuable surrogate tools to ascertain the PBMC activation. Furthermore, treatment of anti-CD3-activated PBMC with increasing concentrations of each of anti-PD1 antibodies (Nivolumab or Pembrolizumab) leads to a significant dose-dependent increase of IFNg release (D), thereby reflecting further optimization of PBMC activation.
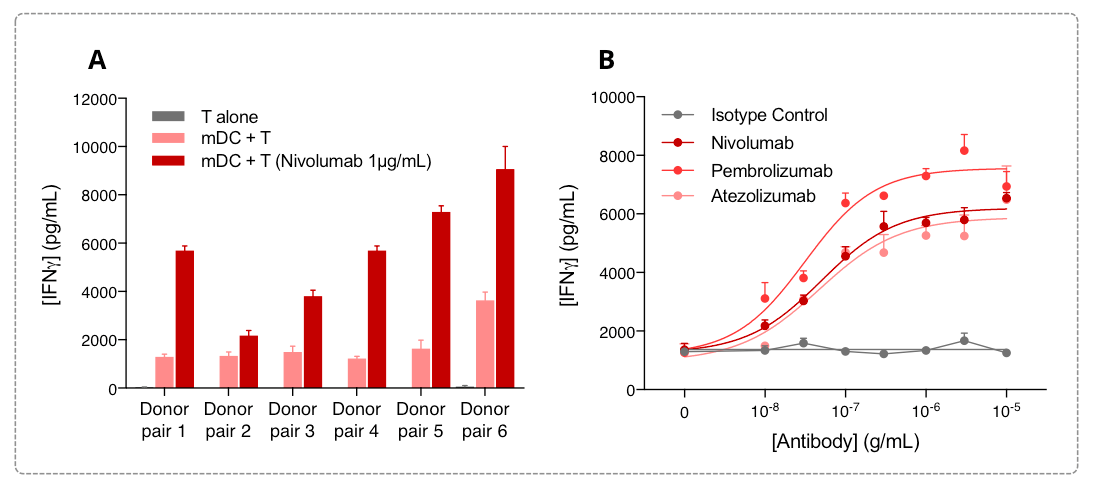
2. T cell IFN𝜸 release is induced in a MLR assay and further optimized by immune checkpoint inhibitors
Our robust and high throughput human allogeneic mixed lymphocyte reaction (MLR) assay consists in a co-culture of CD4+ T cells (responder) with monocyte-derived Dendritic Cells (stimulator). Measurement of released IFNγ constitutes an accurate surrogate to investigate novel immunotherapeutics capable of enhancing DC-mediated T cell activation.
In our assay, isolated peripheral blood monocytes from six human donors are differentiated into mDC and mixed with T CD4+ cells each isolated from different donors (A). IFNg levels released in the supernatants are measured after 72h using a specific HTRF-based specific kit. While displaying an expected response amplitude in IFN𝜸 release varying from a donor pair to another one, the MLR response is robust and is further optimized following PD1 blockade. More thoroughly, PD1/PDL1 blockade dose dependently optimize the MLR response, as highlighted through the dose-dependent promotion of IFN𝜸 release by anti-PD1 (Nivolumab and Pembrolizumab) and anti-PDL1 (Atezolizumab) antibodies , measured after 72h in our allogeneic MLR assay (B).
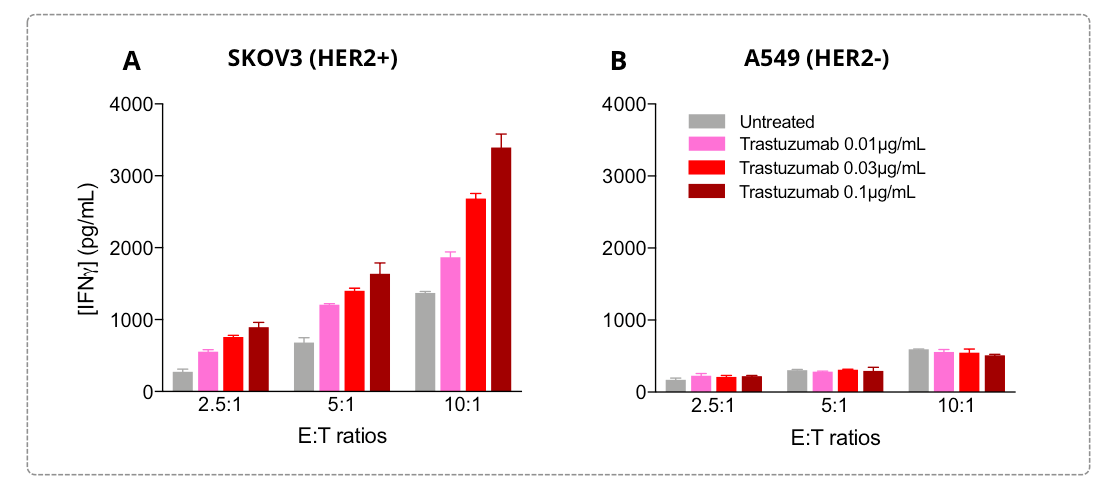
3. IFN𝜸 release by activated NK cells is induced against tumor cells and is optimized by anti-tumor antigen antibodies
In our NK cell-mediated killing assay, we are used to measure the IFN𝜸 release as a functionally relevant surrogate of the NK cell activity / activation to evaluate the efficacy of i) therapeutics in enhancing the NK cell killing ability and of ii) candidate antibodies targeting specific tumor antigens in eliciting an antibody-dependent NK cell cytotoxicity (ADCC) activity.
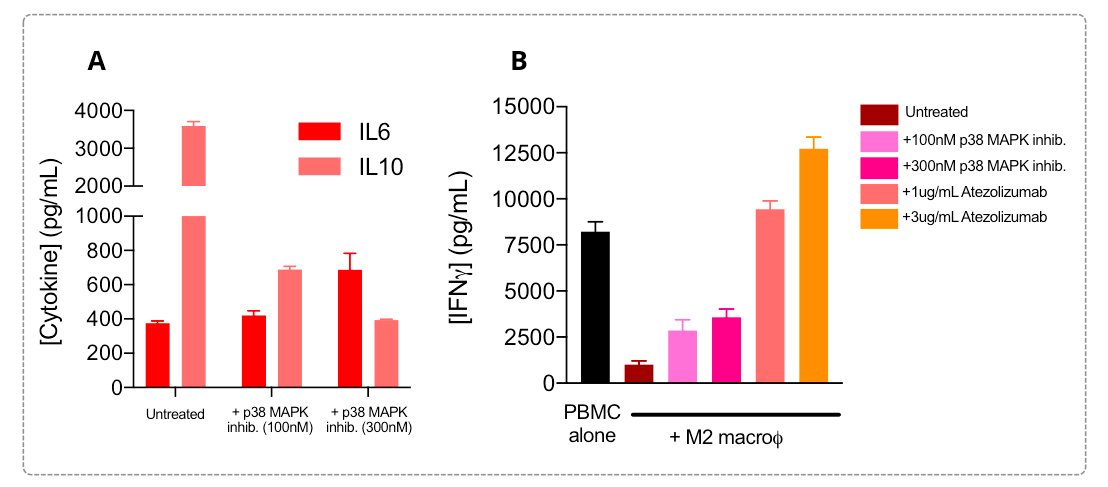
4. M2 macrophage phenotype and its mediated T cell response immunosuppression are reversed by molecular pathway modulators and immune checkpoint inhibitors
M2 macrophages are known to display a IL6low IL10high profile, a phenotype known to be underpinned by ‘’overactivated’’ signaling pathways in these cells, such as JAK/STAT and SMAD/p38 MAPK, and to be therefore involved in their T cell response suppression function.
Addition of a p38 MAPK inhibitor during the M2 polarization process is shown to be capable of repolarizing/switching M2 phenotype by reverting, at least partially but dose-dependently, the IL6low IL10high profile (A). In the co-culture immunosuppression assay with PBMCs (B), M2 macrophages exhibit a strong suppression of T cell activity shown through released IFNg level decrease. M2 suppression function is known to be exerted via multiple mechanisms including PD1/PDL1 axis engagement or pathway modulation, among other ways. Interestingly, co-cultures of activated PBMC with M2 macrophages that underwent treatment with a p38 MAPK inhibitor during their polarization result, at least partially, in a dose-dependent reversal of the T cell activation suppression compared to untreated PBMC/M2 co-cultures. Atezolizumab treatment of co-cultures is also shown to completely and dose-dependently relieve the immunosuppressive M2 function on T cell response, with a reversal of the M2 IFNg release profile.