Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE)
Read our latest paper, titled Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial.
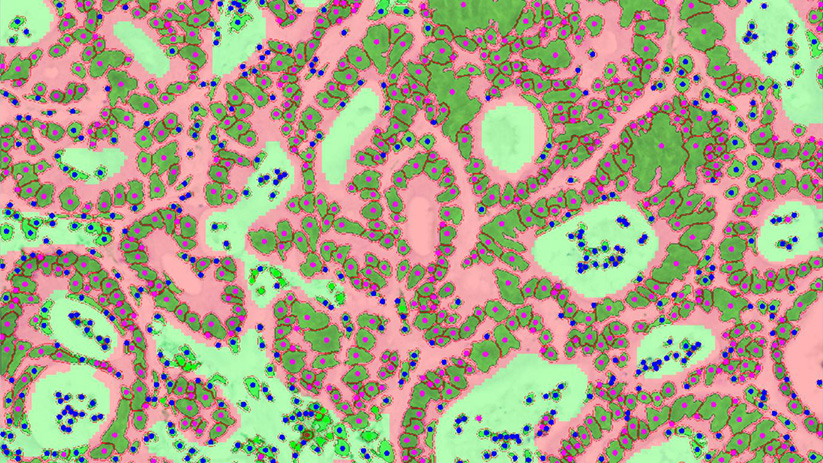
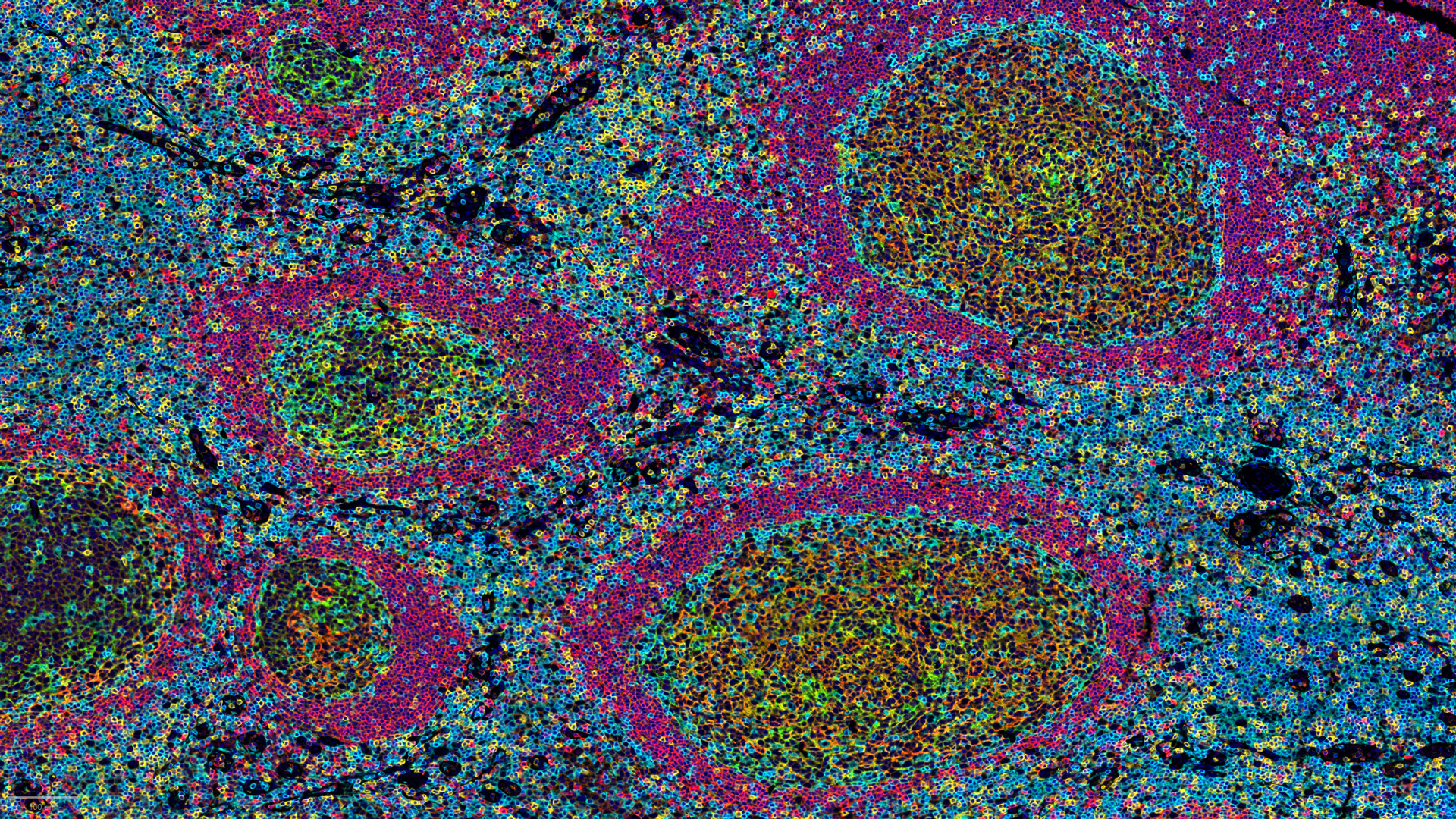
To assess whether or not the tumor immune contexture was predictive of response to the Avelumab-Regorafenib combination regimen, Explicyte developed and ran a multiplexed immunohistofluorescence panel, in addition to Dapi.
It becomes primordial to benefit from a technological platform that allows to deal with the tumor biology complexity/heterogeneity and get specific investigations in compartments of interest (e.g. tumor vs stroma). Thanks to the GeoMx platform (Nanostring Inc.), the Digital Spatial Profiling revolution has started.
To deal with the current challenge of a qualitative and quantitative tumor immune landscape characterization, we have set up a fully integrated platform spanning from FFPE slide preparation and staining, to image digitization and analysis.
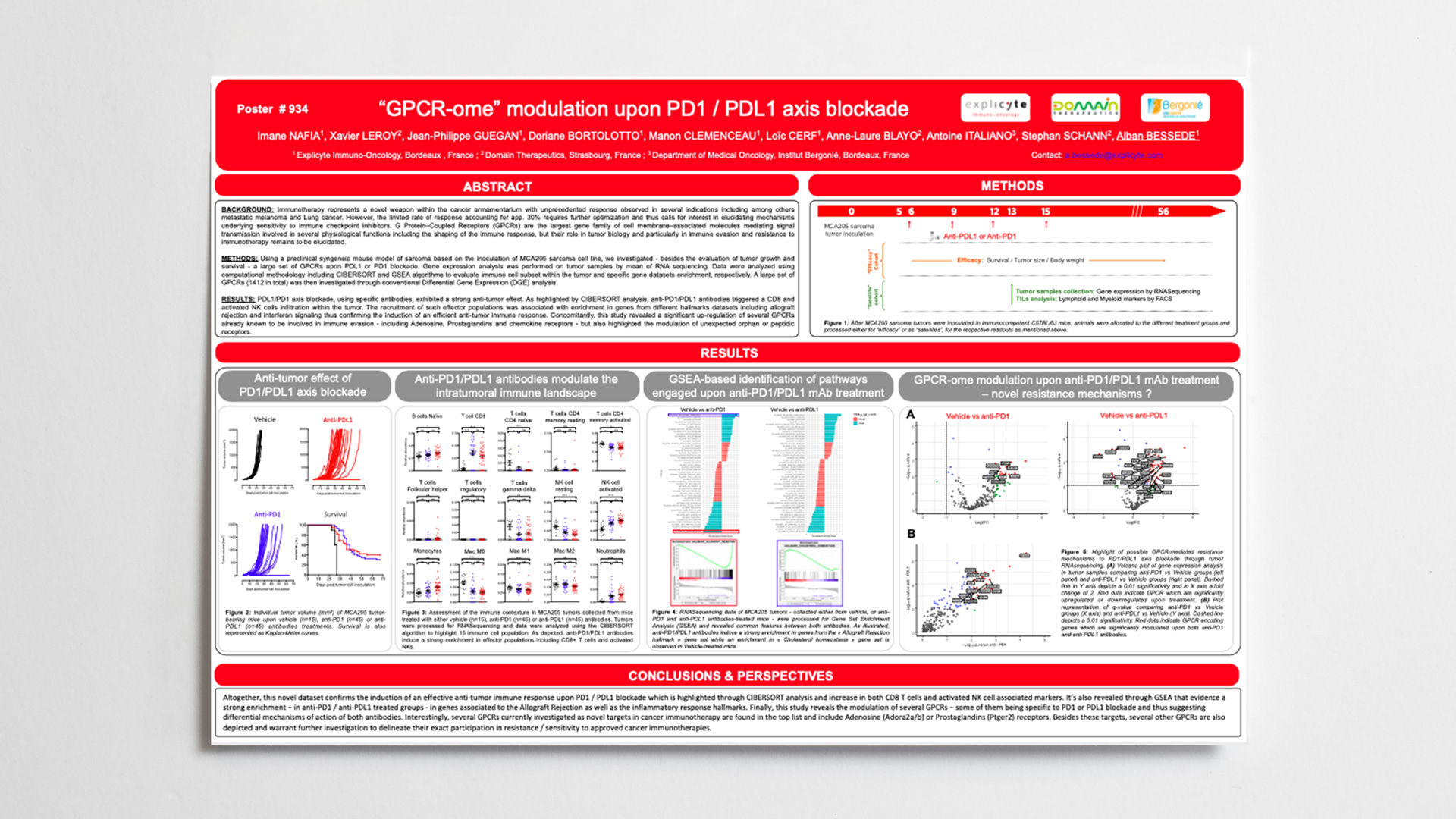
Explicyte will be attending the AACR Virtual Annual Meeting 2020 and will present new results from its collaborative work with Domain Therapeutics on the GPCRome modulation in the context of cancer immunotherapy.
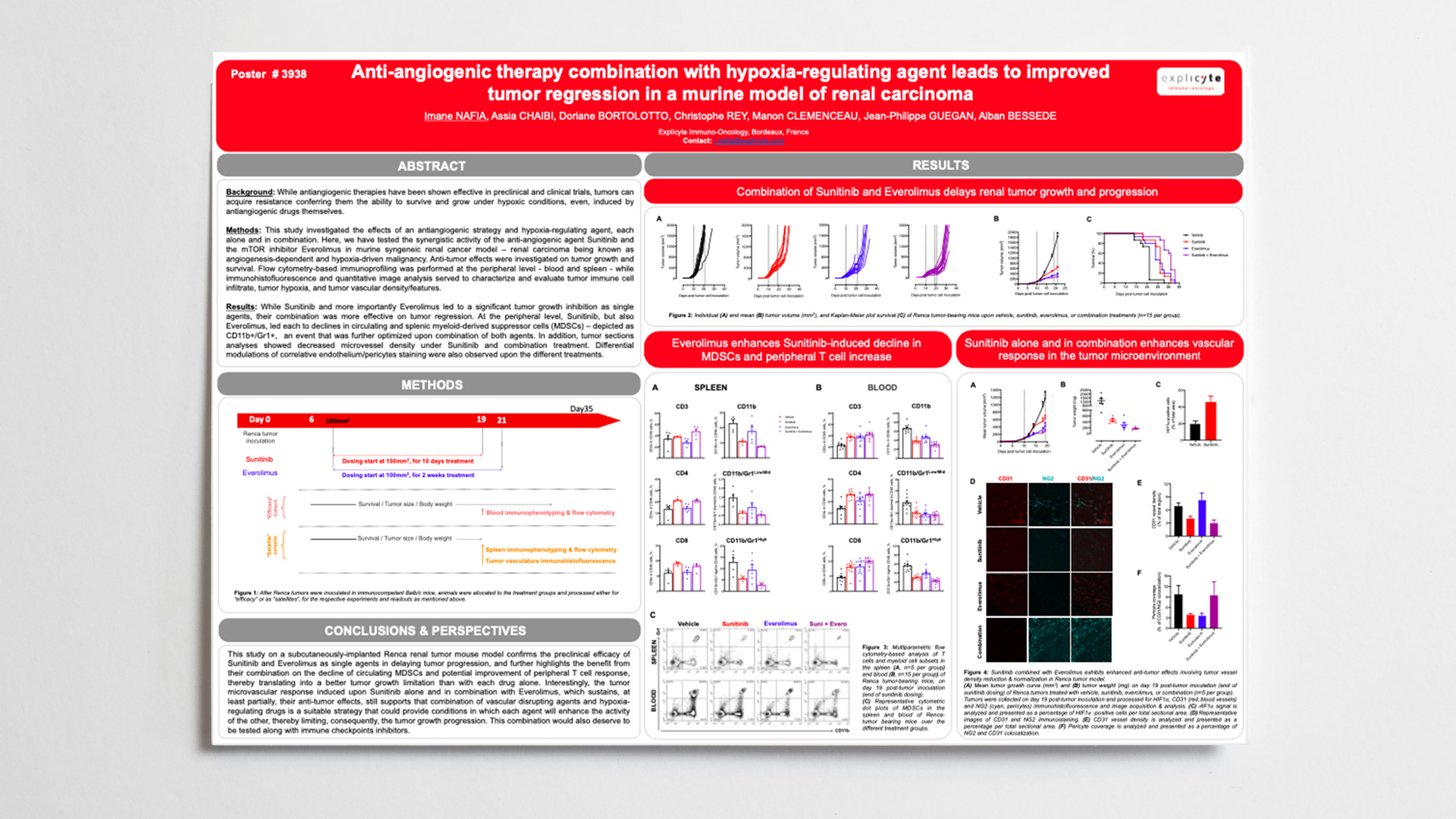
Anti-angiogenic therapy combination with hypoxia-regulating agent leads to improved tumor regression
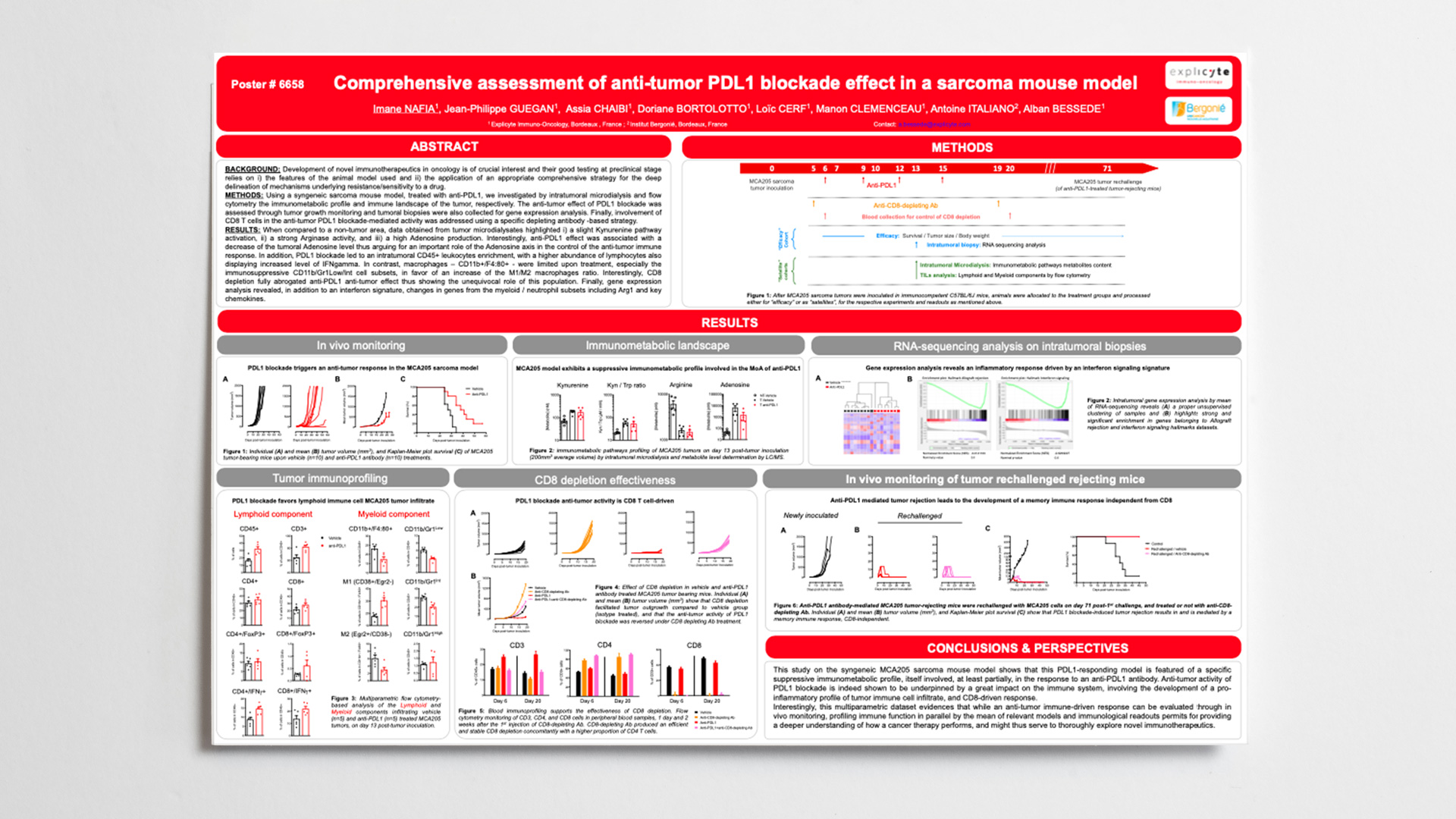
We will be attending the AACR Virtual Annual Meeting, from June 22 to 24, 2020 to present our posters featuring our capacities / platforms that support drug discovery in Immuno-Oncology.
We will be attending the AACR Virtual Annual Meeting, from June 22 to 24, 2020 to present our posters featuring our capacities / platforms that support drug discovery in Immuno-Oncology.
Explicyte’s expertise and advanced technologies have been harnessed so as to build a cell-based service platform for immuno-oncology (IO) assessment and drug discovery.
New explicyte publication in Frontiers in Immunology!
B cells are to the forefront of cancer immunotherapy. Discover how!
How about an opportunity to assess your compounds in a comprehensive and cost-effective manner? Explicyte is planning a new in vivo shuttle session by mid March, to be held on a series of either subcutaneously- or orthotopically-inoculated syngeneic tumor mouse models.