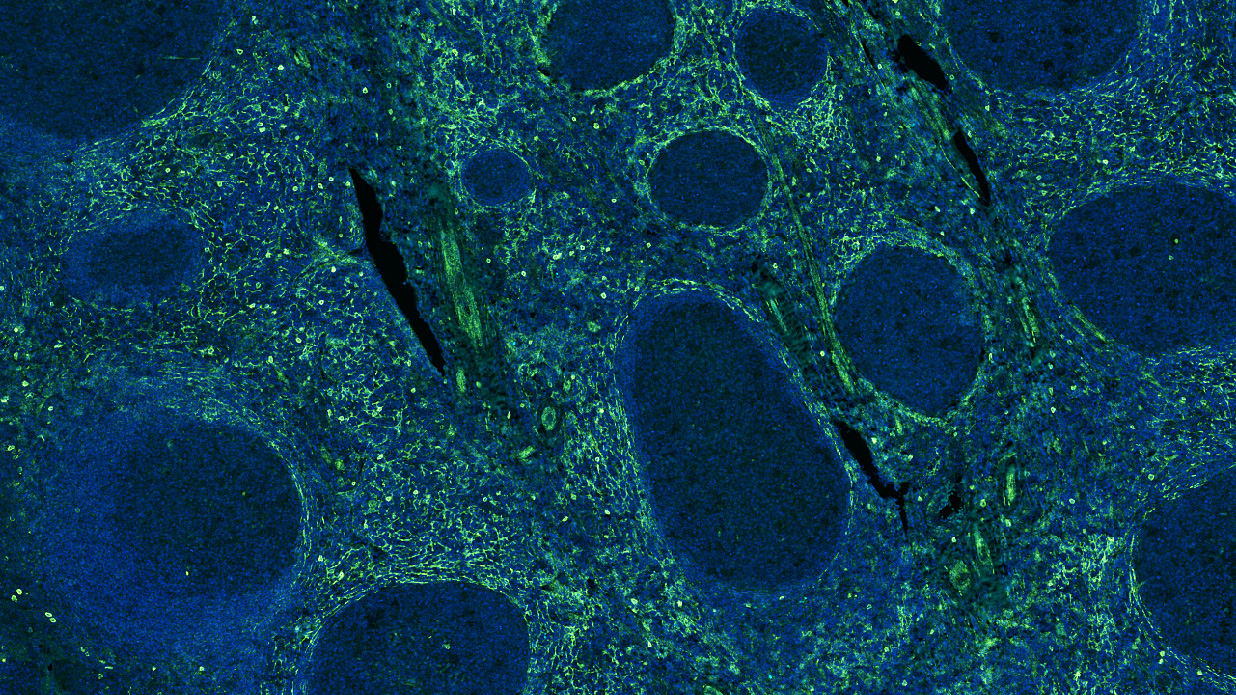
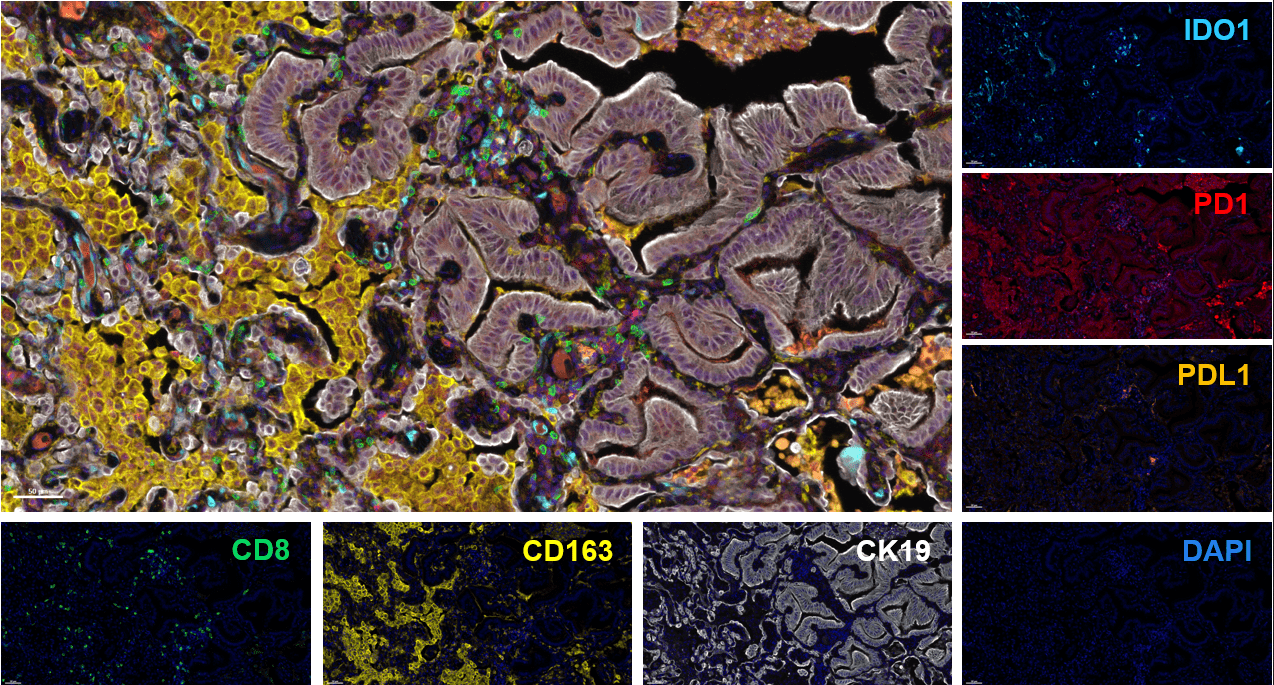
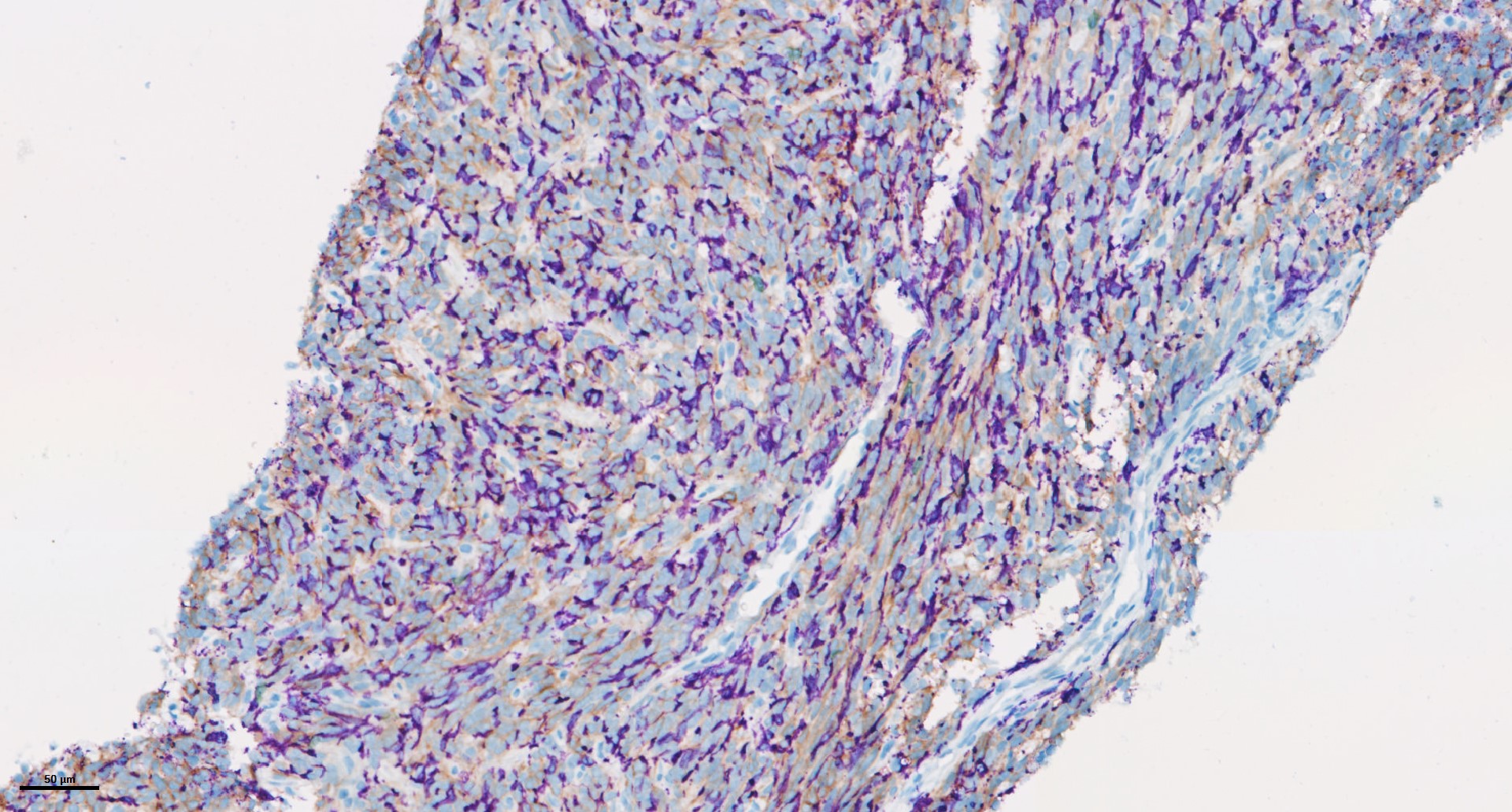
Drawing on our expertise in multiplexed-immunohistofluoresence (IHF) and immunohistochemistry (IHC) assays, we have already developed and validated a series of markers & panels to assess several markers of interest as well as the abundance and spatial distribution of lymphoid and myeloid immune cell subsets. While bringing key answers to address specific questions, our capacities have recently been highlighted in new scientific publications of collaborative works with Institut Bergonié, which we are delighted to share thereafter.
In the era of immuno-oncology (IO) research, there is a tremendous need to i) gain insights into the mechanisms associated with response to immunotherapies, i.e. sensitivity vs resistance, and to ii) identify novel biomarkers that can help select patients likely to benefit from such immunotherapies, either as single agents or in combination with novel drug candidates. Analysis of tumor samples using reliable technologies such as multiplexed immunohistofluorescence (IHF) & immunohistochemistry (IHC) combined to ground-breaking image analysis capacities offers a valuable approach to meet these current challenges.
Drawing on our expertise in IHC and IHF assays, we have already developed and validated a series of markers & panels to assess several markers of interest as well as the abundance and spatial distribution of lymphoid and myeloid immune cell subsets. In addition to implementing validated panels, Explicyte provides tailor-made services to specifically meet the project needs. Furthermore, in order to get an accurate and comprehensive picture from the tumor sample – i.e. considering tumor cells and their microenvironment – all images obtained through slide digitization are processed for analysis using a very well-trained workflow.
While bringing key answers to address specific questions, our capacities have recently been highlighted in new scientific publications of collaborative works with the Institut Bergonié, which we are delighted to share thereafter.