We're excited to share this comprehensive review on Tertiary Lymphoid Structures (TLS), highlighting the work of Florent Peyraud, an oncologist from the Institut Bergonié. Florent completed his PhD thesis at Explicyte under the guidance of Prof. Antoine Italiano (Institut Bergonié/Gustave Roussy). Congratulations, Florent!
This review delves into recent advancements in TLS research, covering:
Our translational team, who co-authored this review, has developed specialized expertise in the detection and scoring of tertiary lymphoid structure (TLS), combining digital pathology and pathologist-assisted image analysis.
Read the article
This review delves into recent advancements in TLS research, covering:
- TLS composition, maturation status, and prevalence across tumor types
- The role of TLS in antitumor immunity and prognosis, including their predictive value in response to immunotherapy
- Therapeutic strategies: drivers of TLS formation, relevant preclinical models, and the induction of TLS using cytokines, chemokines, or existing cancer treatments.
Our translational team, who co-authored this review, has developed specialized expertise in the detection and scoring of tertiary lymphoid structure (TLS), combining digital pathology and pathologist-assisted image analysis.
Read the article
You're developing a small molecule, a biologic (mAb, bispecific, fusion protein), or a vaccination or probiotic-based strategy in immuno-oncology?
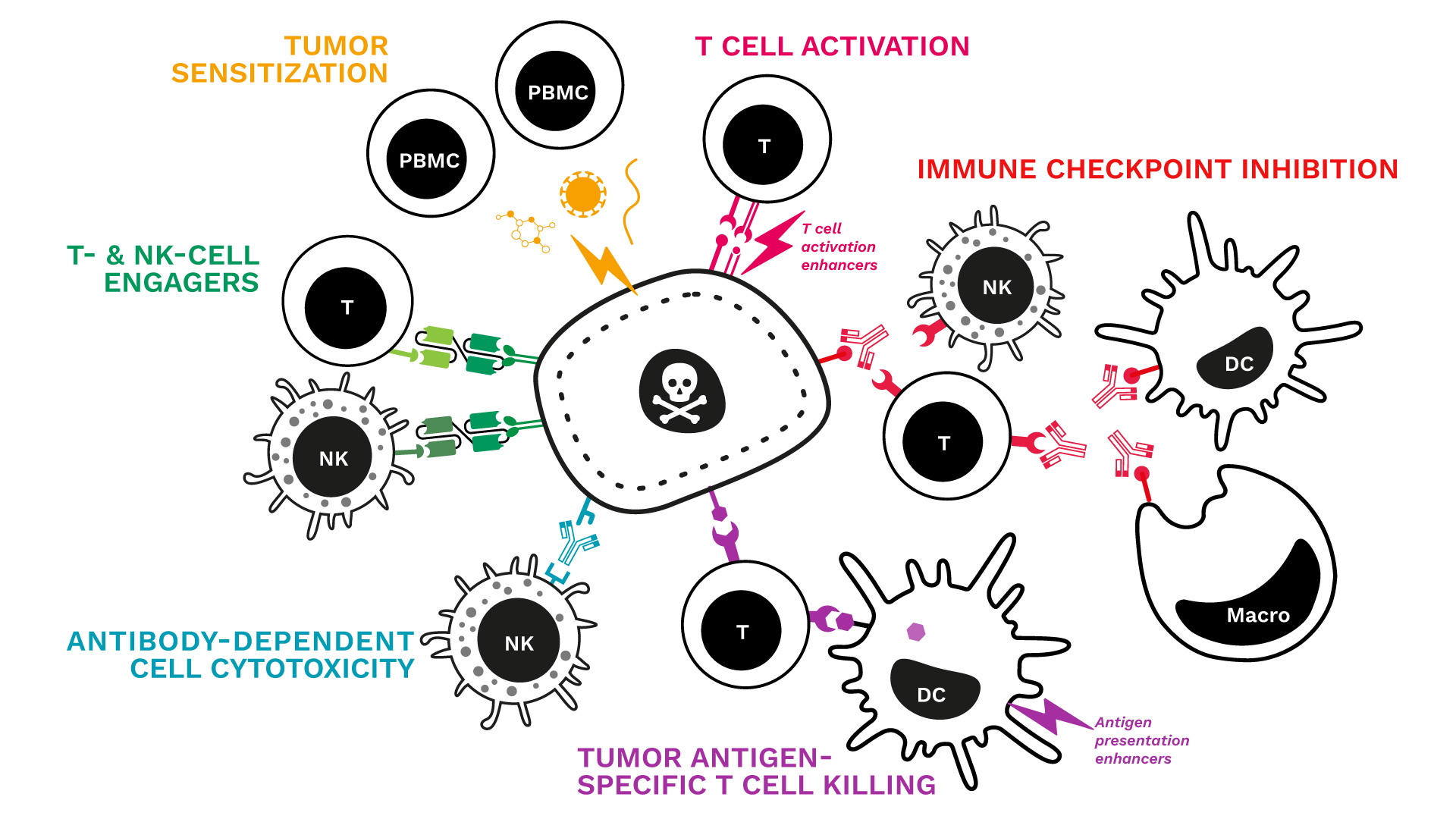
We just updated our range of immune-mediated tumor killing assays!
Key features:
See our specialized killing assays and related data:
We just updated our range of immune-mediated tumor killing assays!
Key features:
- Human co-cultures: the cancer cell line of your choice is cultured with primary immune cells (PBMCs or specific subsets such as Ts or NKs)
- Tumor cell death monitored over time by live-cell imaging
- Immune response captured through the measurement of key cytokines
- A comprehensive analytical platform to further explore and document the profile and mechanism of action of your candidate
See our specialized killing assays and related data:
- Tumor sensitization & killing
- T-cell activation & tumor killing
- Immune checkpoint blockade & tumor killing
- Tumor-antigen specific T cell killing
- Antibody-dependent cell cytotoxicity (NK tumor killing)
A question? A specific request? Contact us !
This retrospective work, led by Prof. Antoine Italiano (Bergonié Comprehensive Cancer Center) and funded by AstraZeneca and the Nouvelle-Aquitaine Regional Council, is based on the BIP study (NCT02534649). It seeks to evaluate the predictive value of CD8+ T cell exhaustion in lung adenocarcinoma patients treated with immune checkpoint inhibitors.
Assessment of TIL Exhaustion by Multiplex Immunohistofluorescence (mIHF)
Explicyte applied a panel indicative of tumor-infiltrating lymphocyte (TIL) exhaustion (CD8, CK7, LAG3, PD1, TIGIT, and TIM3) to tumor samples from 166 patients with advanced lung adenocarcinoma, collected before their treatment with anti-PD1/PD-L1 immunotherapy. Our mIHF analysis revealed that non-responders featured a high proportion of CD8+ T cells co-expressing PD1 and at least one other marker of exhaustion (LAG3, TIGIT, or TIM3). This profile was associated with poorer clinical outcomes, independently of other factors such as age, gender, and PD-L1 status assessed by IHC.
Whole Transcriptome Analysis Reveals a 25-Gene Signature Predictive of CD8+ T Cell Exhaustion and Response to Immunotherapy
Our translational team then analyzed a total of 135 FFPE samples - characterized for their exhaustion status by mIHF - using the HTG transcriptome panel (19,000+ targets). From this analysis, we identified a 25-gene transcriptomic signature strongly associated with the exhaustion phenotype, demonstrating high predictive accuracy. The robustness of this signature was validated in external datasets from NSCLC clinical trials (POPLAR [NCT02517892], OAK [NCT0200822], and MATCH-R [NCT02517892]).
The Predictive Value of the 25-Gene Exhaustion Signature Extends Beyond Lung Cancer
Datasets from the CA209-038 melanoma trial (NCT01621490) and the JAVELIN Renal 101 (NCT0268400601) trial in renal carcinoma confirmed the robustness of the CD8+ T cell exhaustion signature as a biomarker for stratifying patients likely to benefit from immunotherapy.
Assessment of TIL Exhaustion by Multiplex Immunohistofluorescence (mIHF)
Explicyte applied a panel indicative of tumor-infiltrating lymphocyte (TIL) exhaustion (CD8, CK7, LAG3, PD1, TIGIT, and TIM3) to tumor samples from 166 patients with advanced lung adenocarcinoma, collected before their treatment with anti-PD1/PD-L1 immunotherapy. Our mIHF analysis revealed that non-responders featured a high proportion of CD8+ T cells co-expressing PD1 and at least one other marker of exhaustion (LAG3, TIGIT, or TIM3). This profile was associated with poorer clinical outcomes, independently of other factors such as age, gender, and PD-L1 status assessed by IHC.
Whole Transcriptome Analysis Reveals a 25-Gene Signature Predictive of CD8+ T Cell Exhaustion and Response to Immunotherapy
Our translational team then analyzed a total of 135 FFPE samples - characterized for their exhaustion status by mIHF - using the HTG transcriptome panel (19,000+ targets). From this analysis, we identified a 25-gene transcriptomic signature strongly associated with the exhaustion phenotype, demonstrating high predictive accuracy. The robustness of this signature was validated in external datasets from NSCLC clinical trials (POPLAR [NCT02517892], OAK [NCT0200822], and MATCH-R [NCT02517892]).
The Predictive Value of the 25-Gene Exhaustion Signature Extends Beyond Lung Cancer
Datasets from the CA209-038 melanoma trial (NCT01621490) and the JAVELIN Renal 101 (NCT0268400601) trial in renal carcinoma confirmed the robustness of the CD8+ T cell exhaustion signature as a biomarker for stratifying patients likely to benefit from immunotherapy.
Read paper
Imane Nafia, PhD, Chief Scientific Officer of Explicyte, will attend AACR IO in Los Angeles (Feb. 23-26).
Contact us to arrange a meeting on-site
Contact us to arrange a meeting on-site
In this retrospective study led by Pr. Antoine Italiano (Bergonié & Gustave Roussy Comprehensive Cancer Centers) based on the NEOSARCOMICS trial, we investigated whether microenvironment features of undifferentiated pleomorphic sarcomas (UPS) - an aggressive subtype of soft tissue sarcoma - could predict response to neoadjuvant anthracycline-based chemotherapy.
These results suggest that immune infiltration status can serve to stratify UPS patients before surgical resection. Patients with high immune infiltration benefit from a better prognosis from the start and may respond poorly to chemotherapy. However, neoadjuvant therapy may improve the prognosis of immune-low UPS patients.
- Microenvironment features & prognosis without neoadjuvant therapy: We applied a multiplexed immunohistofluorescence (mIHF) panel on tumor samples from 47 patients with UPS who underwent surgical resection. We found that patients with poor survival had fewer infiltrated immune cells (especially CD8+ cells and M1 macrophages), while patients with better overall survival tended to feature high tumor infiltration.
- Microenvironment features & response to neoadjuvant therapy: We then analyzed the gene expression of baseline samples from 24 patients with resectable UPS, who were treated with neoadjuvant chemotherapy. We found that a good response was associated with a high proliferation and low immune infiltration phenotype. This finding was confirmed with the multiplex mIHF panel: patients who poorly respond to neoadjuvant therapy feature high immune infiltration. Plasma proteomics confirmed the upregulation of cell cycle pathways in low immune infiltration patients.
These results suggest that immune infiltration status can serve to stratify UPS patients before surgical resection. Patients with high immune infiltration benefit from a better prognosis from the start and may respond poorly to chemotherapy. However, neoadjuvant therapy may improve the prognosis of immune-low UPS patients.
Read paper in the Journal Hematology & Oncology
Explicyte is the first immuno-oncology CRO in Europe equipped with the 10x Xenium platform for single-cell spatial transcriptomics! Training by the 10x Genomics team started! Why is it exciting for immuno-oncology?
“For our translational and data science team, the Xenium platform opens unexplored avenues for analyzing tumors and their microenvironment in response to various cancer modalities, including immunotherapies.
For our sponsors, gaining a deep understanding of the gene expression profiles and spatial distribution of individual cells within a tumor specimen can provide unique insights into understanding tumor biology, and mechanisms at play in anti-cancer response or resistance to treatment. It creates opportunities to identify new targets and biomarkers to predict clinical outcomes for cancer patients.
As a researcher, I’m eager to watch tumor cells, immune cells, cancer-associated fibroblasts with the Xenium eye, and to witness the single-cell architecture of immune infiltrates and tertiary lymphoid structures. We are truly excited as we enter a new era of cancer discovery."
Alban Bessede, CEO of Explicyte Immuno-Oncology.
About the 10x Xenium platform:
Learn about our services for target identification & biomarker discovery in immuno-oncology.
“For our translational and data science team, the Xenium platform opens unexplored avenues for analyzing tumors and their microenvironment in response to various cancer modalities, including immunotherapies.
For our sponsors, gaining a deep understanding of the gene expression profiles and spatial distribution of individual cells within a tumor specimen can provide unique insights into understanding tumor biology, and mechanisms at play in anti-cancer response or resistance to treatment. It creates opportunities to identify new targets and biomarkers to predict clinical outcomes for cancer patients.
As a researcher, I’m eager to watch tumor cells, immune cells, cancer-associated fibroblasts with the Xenium eye, and to witness the single-cell architecture of immune infiltrates and tertiary lymphoid structures. We are truly excited as we enter a new era of cancer discovery."
Alban Bessede, CEO of Explicyte Immuno-Oncology.
About the 10x Xenium platform:
- Compatible with standard tumor specimens (frozen & FFPE)
- TME architecture is visualized at subcellular level with a 5000-gene panel
- Single-cell analysis & segmentation with AI-driven algorithm
Learn about our services for target identification & biomarker discovery in immuno-oncology.
In this study led by Pr. Antoine Italiano (Bergonié & Gustave Roussy Comprehensive Cancer Centers), 49 patients with advanced gastric cancer were treated with avelumab (a PD-1/PD-L1 inhibitor) in combination with the anti-angiogenic drug regorafenib (a multi-kinase tyrosine inhibitor). Despite encouraging efficacy results, a significant subset of patients did not respond to this therapeutic regimen.
Explicyte utilized spatial transcriptomics to profile the expression of over 18,000 protein-coding genes across six tumors from both responding and non-responding patients from the REGOMUNE & REGONIVO studies (both combining regorafenib with immune checkpoint inhibition). In the immune compartments of resistant patients, the CD163 gene was found to be overexpressed, alongside an enrichment in M2 macrophages. Non-responding patients also exhibited a strong upregulation of the S100A10 protein in tumor cells—a protein involved in macrophage chemotaxis.
We then developed two immunohistofluorescence (IHF) panels to validate these findings via digital pathology in 43 tumor biopsies. The results confirmed the abundance of M2 macrophages in resistant patients, with a significant increase in the M2/M1 ratio, and the overexpression of S100A10 in tumor cells among patients with poor responses.
Finally, plasma biomarkers were investigated using an Olink proteomic panel. Several cytokines (CSF-1, IL-4, IL-8, and TWEAK) associated with macrophage infiltration were found to be upregulated in patients with worse outcomes.
Altogether, this paper highlights the central role of M2 macrophages in the resistance to anti-PD-1/PD-L1 immunotherapy combined with anti-angiogenic therapy in gastric cancers and provides perspectives for novel diagnostic and therapeutic approaches.
Explicyte utilized spatial transcriptomics to profile the expression of over 18,000 protein-coding genes across six tumors from both responding and non-responding patients from the REGOMUNE & REGONIVO studies (both combining regorafenib with immune checkpoint inhibition). In the immune compartments of resistant patients, the CD163 gene was found to be overexpressed, alongside an enrichment in M2 macrophages. Non-responding patients also exhibited a strong upregulation of the S100A10 protein in tumor cells—a protein involved in macrophage chemotaxis.
We then developed two immunohistofluorescence (IHF) panels to validate these findings via digital pathology in 43 tumor biopsies. The results confirmed the abundance of M2 macrophages in resistant patients, with a significant increase in the M2/M1 ratio, and the overexpression of S100A10 in tumor cells among patients with poor responses.
Finally, plasma biomarkers were investigated using an Olink proteomic panel. Several cytokines (CSF-1, IL-4, IL-8, and TWEAK) associated with macrophage infiltration were found to be upregulated in patients with worse outcomes.
Altogether, this paper highlights the central role of M2 macrophages in the resistance to anti-PD-1/PD-L1 immunotherapy combined with anti-angiogenic therapy in gastric cancers and provides perspectives for novel diagnostic and therapeutic approaches.
Read paper in Molecular Cancer
Since 2021, Explicyte has developed a specific expertise in Tertiary Lymphoid Structure (TLS) detection and scoring by digital pathology. Hosted by Alban Bessede, PhD, CEO of Explicyte, this 45-minute webinar dedicated to TLS modulation in cancer immunotherapy will feature 2 presentations:
Following a PhD thesis on intratumoral T cells at Johns Hopkins in 2010, Tullia Bruno started working on the role of B cells in the TME during her postdoctoral fellowship at the University of Colorado Denver. In 2015, she joined the University of Pittsburgh as an Assistant Professor to investigate the role of intratumoral B cell function within TLS in solid tumors. Focused on translational research and the development of novel immunotherapies, she is also a faculty member in the Tumor Microenvironment Center and the Cancer Immunology and Immunotherapy Program at the UPMC Hillman Cancer Center.
Author of 500+ peer-reviewed publications, Pr. Italiano has been the Principal Investigator of 50+ clinical trials over the past 5 years, investigating the resistance mechanisms to cancer immunotherapies in solid tumors. Trained as a medical oncologist in France, he obtained a PhD in Molecular & Cell Biology in 2008. He then joined the laboratory of Cristina Antonescu for a post-doctoral fellowship at the MSKCC (New York, USA). He is currently leading Early Phase Trials and Sarcoma Units at Institut Bergonié (Bordeaux, France) and the head of the Precision Medicine unit at Gustave Roussy (Villejuif, France).
More about Explicyte's capacities for TLS detection & scoring in patient specimens
Tertiary lymphoid structures as modulators of anti-tumor immunity: what do we know and where are we headed?
Tullia C. Bruno, PhD - UPMC Hillman Cancer Center / University of Pittsburgh
Following a PhD thesis on intratumoral T cells at Johns Hopkins in 2010, Tullia Bruno started working on the role of B cells in the TME during her postdoctoral fellowship at the University of Colorado Denver. In 2015, she joined the University of Pittsburgh as an Assistant Professor to investigate the role of intratumoral B cell function within TLS in solid tumors. Focused on translational research and the development of novel immunotherapies, she is also a faculty member in the Tumor Microenvironment Center and the Cancer Immunology and Immunotherapy Program at the UPMC Hillman Cancer Center.
Predictive role of TLS for cancer immunotherapy: current insights and perspectives
Pr Antoine Italiano, MD, PhD - Institut Bergonié & Gustave Roussy Comprehensive Cancer Centers
Author of 500+ peer-reviewed publications, Pr. Italiano has been the Principal Investigator of 50+ clinical trials over the past 5 years, investigating the resistance mechanisms to cancer immunotherapies in solid tumors. Trained as a medical oncologist in France, he obtained a PhD in Molecular & Cell Biology in 2008. He then joined the laboratory of Cristina Antonescu for a post-doctoral fellowship at the MSKCC (New York, USA). He is currently leading Early Phase Trials and Sarcoma Units at Institut Bergonié (Bordeaux, France) and the head of the Precision Medicine unit at Gustave Roussy (Villejuif, France).
More about Explicyte's capacities for TLS detection & scoring in patient specimens
Register for the webinar
In soft tissue sarcomas, the presence of tertiary lymphoid structures (TLS) is known to increase sensitivity to immune checkpoint inhibitors. In a clinical trial conducted at the Institut Bergonié, Prof. Antoine Italiano and colleagues studied the combination of an oncolytic virus and metronomic chemotherapy with the aim of remodeling the tumor microenvironment of patients with TLS-negative soft-tissue sarcoma, thereby improving their sensitivity to the PD-L1 inhibitor Avelumab.
As part of this study, tumor biopsies and plasma samples were supported and processed by Explicyte teams, for staining and Olink-based proteomics, respectively. Our results confirm that intratumoral administration of the oncolytic virus JX-584 can alter the immune landscape of the tumor microenvironment, potentially converting cold tumors into immunogenic tumors.
As part of this study, tumor biopsies and plasma samples were supported and processed by Explicyte teams, for staining and Olink-based proteomics, respectively. Our results confirm that intratumoral administration of the oncolytic virus JX-584 can alter the immune landscape of the tumor microenvironment, potentially converting cold tumors into immunogenic tumors.
Read the article
New paper : Faecalibaterium prausnitzii strain EXL01 boosts efficacy of immune checkpoint inhibitors
We're happy to showcase a collaboration just published in OncoImmunology. Since 2016, French biotech Exeliom Bioscience has been advancing therapeutic programs targeting the gut microbiota in Crohn's disease and various types of cancer, based on the immuno-modulatory bacterium Faecalibacterium prausnitzii (oral drug EXL01). In this paper, we supported Exeliom Bioscience in the generation of in vivo and vitro data to decipher the mechanism of action of EXL01.
The in vivo study, based on the MCA205 syngeneic tumor model, demonstrates that oral supplementation with EXL1 restores the efficacy of anti-PD-L1 immunotherapy in animals with antibiotics-induced gut dysbiosis. The subsequent in vitro investigation, based on the MLR assay, showed that the EXL1 strain boosts the activation of both dendritic cells and T cells in the presence of anti-PD-L1 antibody.
The in vivo study, based on the MCA205 syngeneic tumor model, demonstrates that oral supplementation with EXL1 restores the efficacy of anti-PD-L1 immunotherapy in animals with antibiotics-induced gut dysbiosis. The subsequent in vitro investigation, based on the MLR assay, showed that the EXL1 strain boosts the activation of both dendritic cells and T cells in the presence of anti-PD-L1 antibody.