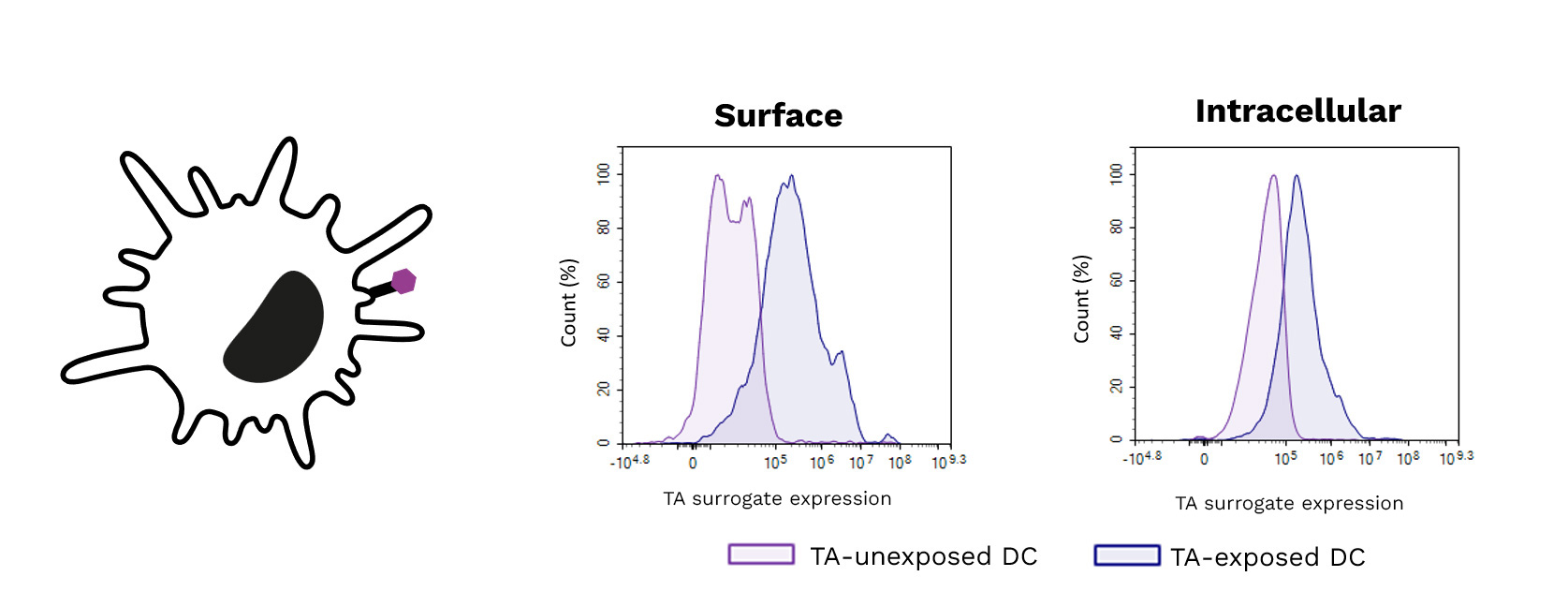
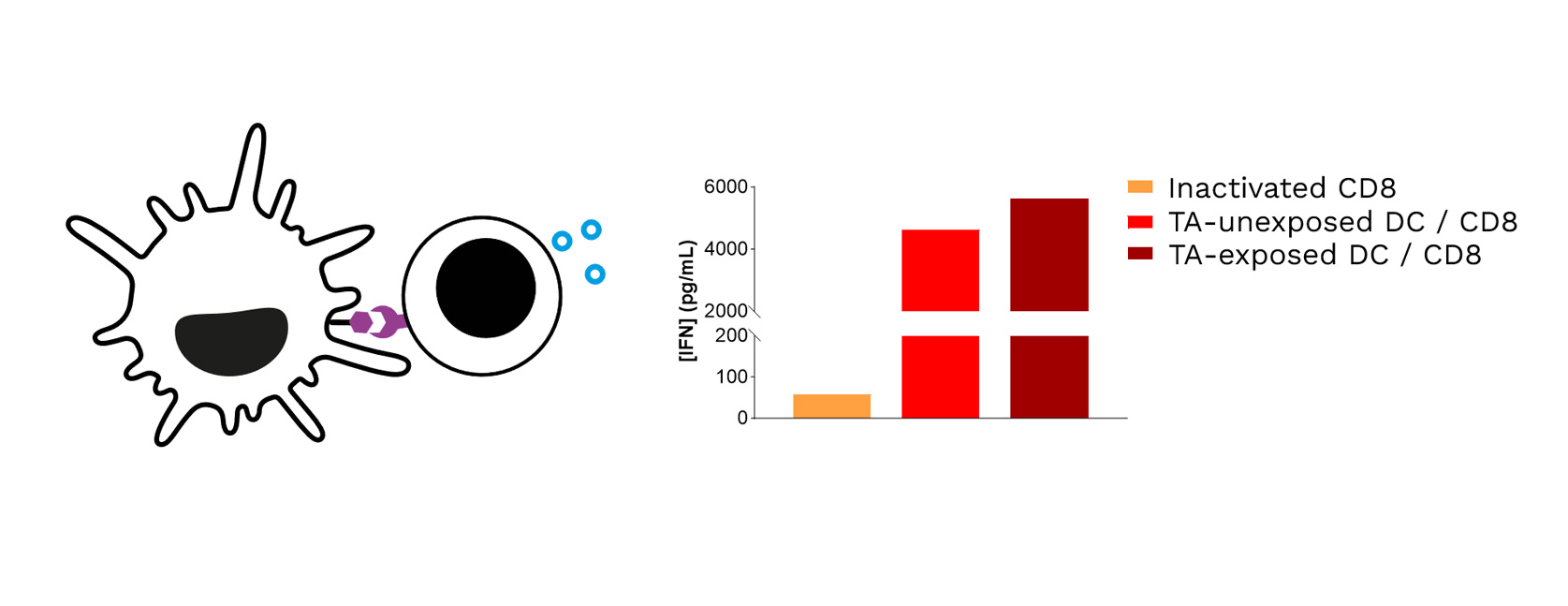
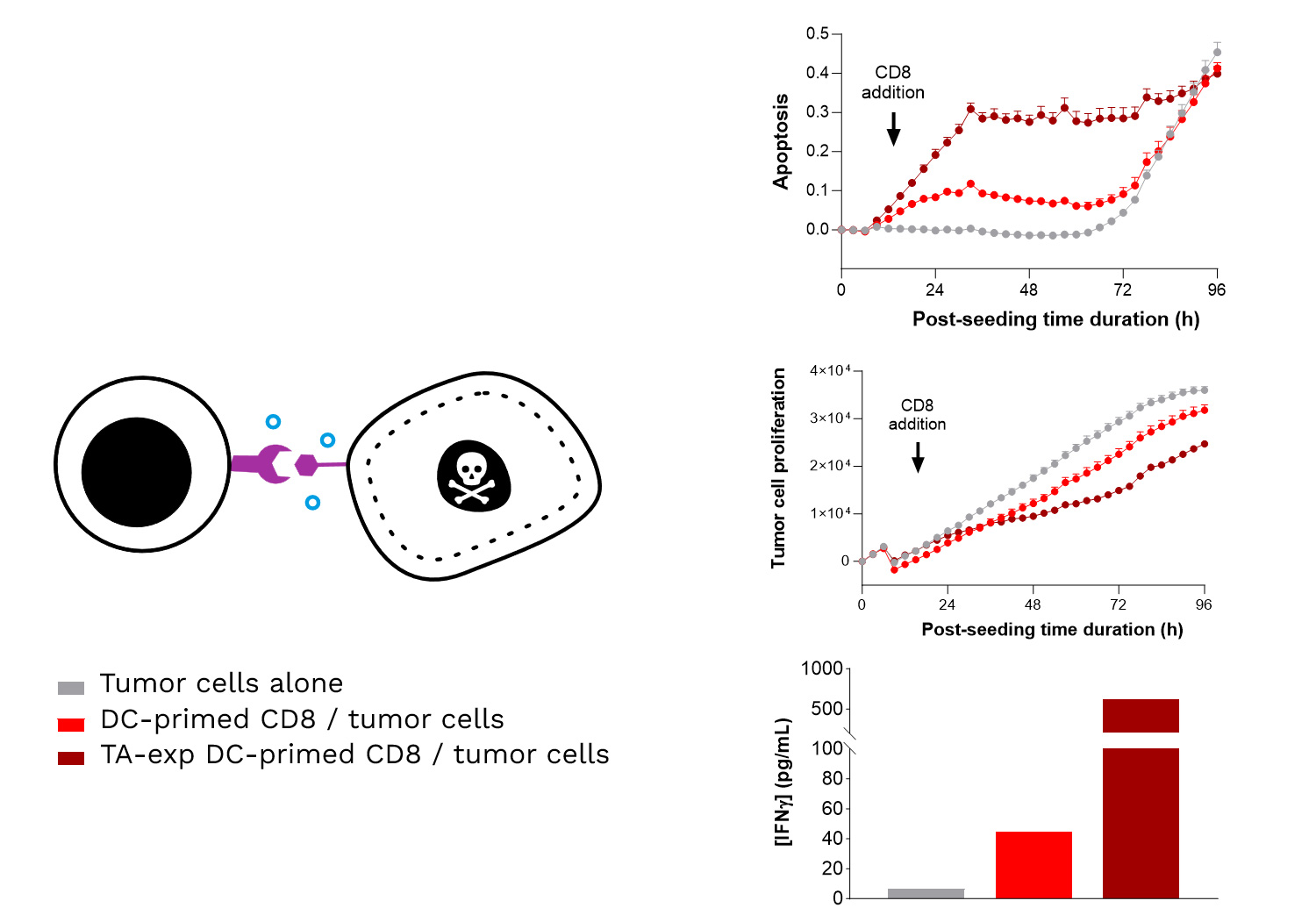
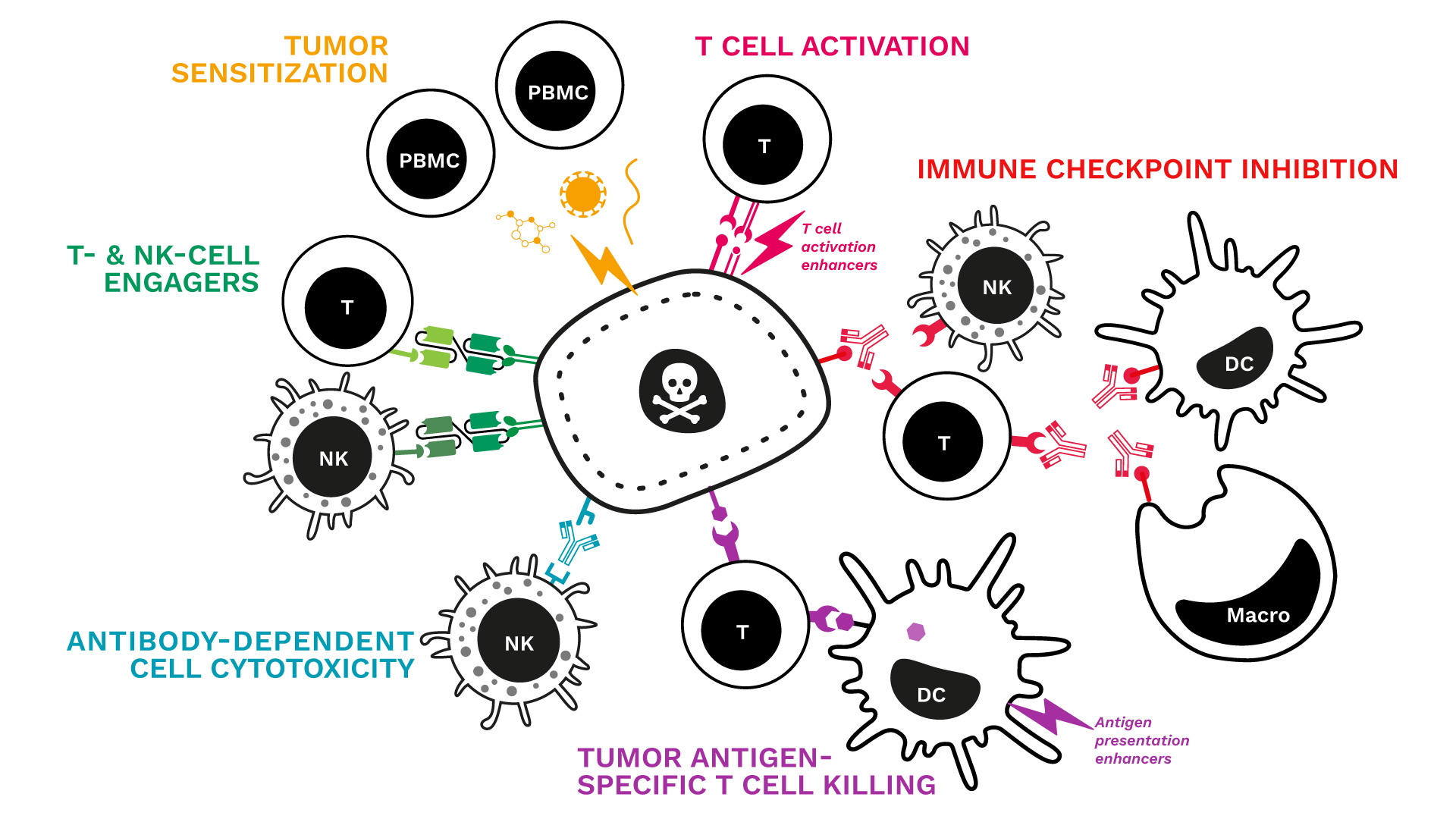
- Over the past two years, Cure51 has collected tumor samples from 1000 exceptional cancer survivors in collaboration with 100+ leading cancer centers worldwide.
- A first batch of tumor samples, processed and qualified at Institut Gustave Roussy in Paris, is being transferred in June 2025 to Explicyte—a Bordeaux-based CRO dedicated to precision oncology with unique capacities in single-cell spatial transcriptomics in Europe
PARIS, FRANCE, 26 June, 2025 - French TechBio startup company Cure51 set to unlock the biological mechanisms from cancer survivors, and precision oncology CRO Explicyte announced today a strategic partnership to analyze tumor samples from exceptional cancer survivors using single-cell spatial transcriptomic technologies. The resulting gene expression maps, generated at single-cell resolution, will be analyzed by Cure51’s data science team to uncover the molecular drivers of long-term survival and identify new therapeutic strategies.
Launched in November 2023, the Cure51’s Rosalind study seeks to decode the biological mechanisms of super responders—patients who defy the odds by surviving highly aggressive cancers—in collaboration with leading cancer centers around the world. The study targets three aggressive cancer types, Small Cell Lung Cancer (led by Charité, Berlin, Germany), Glioblastoma (led by Leon Berard, Lyon, France), and Metastatic pancreatic cancer (led by Gustave Roussy, Paris, France).
Cure51 has built a unique global network, partnering with 100 cancer centers to collect tumor samples and clinical data from over 1.000 cancer patients from 40 countries. The company’s mission is to create an unparalleled multi-omics database to drive the development of novel cancer therapies.
Leveraging Explicyte´s expertise in precision oncology and capitalizing on their deep experience of 10x genomics spatial tools and technology, Cure51 intends to accelerate its discovery process by better understanding the biology of Exceptional Survivors.
"At Cure51, we are reverse engineering the cure for cancer. By leveraging technology, data and our dedicated team of computational biologists, we aim to uncover the hidden biology of miraculous survivors, to develop therapies that could one day make cancer a manageable disease for all, “ said Cure51 co-Founders Nicolas Wolikow and Simon Istolainen. “ In just 18 months, our team forged international partnerships to secure access to tumor samples and clinical data from Cancer Outliers. To advance our data generation pipeline, we were looking for an EU-based partner with the scientific rigor and technical capabilities to process these valuable samples efficiently and reproducibly. Explicyte stood out for their strong scientific track record and cutting-edge technological expertise.”
"Over the past five years, first-generation spatial transcriptomics technologies have driven major advances in our understanding of the determinants of immunotherapy response, leading to over 30 co-authored publications with our academic and industry partners. In 2024, we took a significant step forward by establishing a unique single-cell spatial transcriptomics platform in Europe, built on 10x Genomics technologies. Cure51’s outlier-focused cohort is truly one of a kind. We are confident that this collaboration will generate transformative insights in the fight against cancer, and are excited to see Cure51 roll out."
— Alban Bessède, CEO, Explicyte
Earlier this year, Cure51 announced a major partnership with the Hopitaux de Paris (AP-HP), a network of 39 hospitals across France. In March 2024, the company raised €15 million in Seed funding led by Sofinnova Partners, Hitachi Ventures and LifeX. Cure51 is named after the British biochemist Rosalind Franklin, one of the greatest pioneers in medical research. Franklin and her doctoral student Ray Gosling captured the so-called ‘Photo 51’, which enabled the discovery of the double helix structure of DNA in 1953. The company’s motto is Cancer Delenda Est (Cancer is defeated).
About Cure51
Cure51 is a French techbio company founded by Nicolas Wolikow and Simon Istolainen alongside seasoned entrepreneurs and five world-renowned oncology centres: Gustave Roussy Cancer Campus (IGR, Paris - France), Leon Bérard Center (CLB, Lyon - France), Charité Universitätsmedizin Berlin (Germany), and Vall d'Hebron (VHIO, Barcelona, Spain). The collaboration between the private and public sectors is at the core of the Cure51 project, led by a passionate team with expertise in computing, medicine, and biology, and partnered with principal investigators across its network of over 50 leading oncology centres worldwide.
Cure51's exclusive data collection system, based on partnerships, enables the creation of a unique multimodal and multiomics database of Outliers. Using its discovery platform powered by computational modeling, Cure51 aims to understand the biological mechanisms responsible for this exceptional survival by identifying and validating targets that can act on these interactions, leading to first-in-class treatments. This research involves the use of all relevant models (in silico, in vitro, in vivo, ex vivo) and the integration of existing literature and available databases, along with the engagement of Cure51's KOL community. Ultimately, drug design will be subject to collaboration contracts with the industry.
For further information, please visit www.cure51.com
About Explicyte
Explicyte is a preclinical and translational contract research organization specializing in precision oncology. Founded in 2015 by immunologist Dr. Alban Bessède, the company has supported more than 100 biotech and pharmaceutical partners over the past decade in the discovery and development of novel therapies for solid tumors. Based at the Institut Bergonié Comprehensive Cancer Center in Bordeaux, Explicyte brings together a multidisciplinary team of 25 scientists, including cell biologists, digital pathologists, medical oncologists, and data scientists. In the past five years, Explicyte has co-authored over 30 peer-reviewed publications on the molecular mechanisms underlying responses to cancer immunotherapies.
For more information, visit www.explicyte.com
Press contacts
Cure51 – Clara Armand-Delille clara@thirdeyemedia.press
Explicyte – Pierre-Emmanuel GAULTIER - pe.gaultier@explicyte.com - +33 6 450 600 49
Download press kit (photos, bios, etc...)