How about an opportunity to assess your compounds in a comprehensive and cost-effective manner?
Explicyte is planning a new in vivo shuttle session by mid March, to be held on a series of either subcutaneously– or orthotopically-inoculated syngeneic tumor mouse models. These models can be run either for only efficacy monitoring or for a comprehensive assessment based on a multiparametric platform strategy for a deeper understanding of how a cancer therapy performs.
For this shuttle session, registration is now open, with a deadline on February 21, 2020.
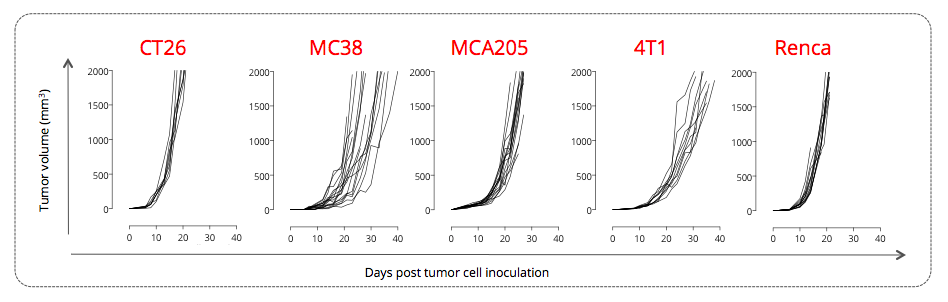
Figure 1: Subcutaneously-implanted CT26 colon, MC38 colon, MCA205 sarcoma, 4T1 breast, and Renca renal tumor-bearing mouse models trigger various tumor growth profiles. Mice were subcutaneously implanted with respective tumor cells and tumor growth was monitored overtime via calipering. Tumor volume and survival were then determined.
Case study 1 – Subcutaneously-inoculated MCA205 sarcoma mouse tumor model
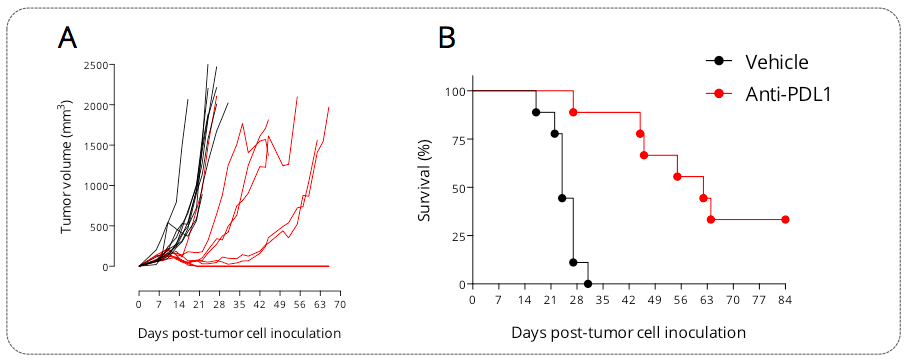
Figure 2: Subcutaneously-implanted MCA205 sarcoma tumor-bearing mouse model is responsive to PDL1 blockade. Mice were subcutaneously implanted with tumor cells and exposed to anti-PDL1 antibody. Tumor growth was monitored overtime via calipering. Tumor volume (A) and survival (B) were then determined.
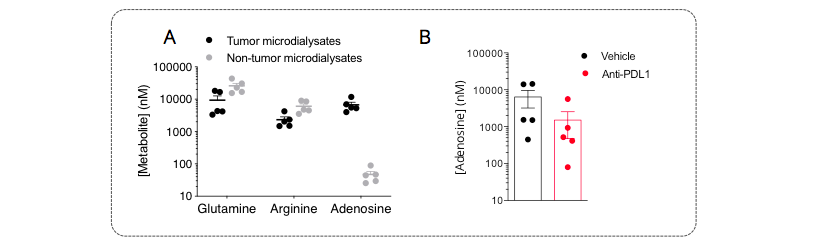
Figure 3: A. Immunometabolic profiling of the MCA205 sarcoma model by intratumoral microdialysis. Mice were implanted with tumor cells and then processed for intratumoral microdialysis at the tumor volume average of ~200mm3, for the determination of glutamine, arginine, and adenosine levels by LC/MS. The tumor model displays differential levels for arginine and particularly for adenosine between tumor and non-tumor compartments – both pathways are known to be involved in the immune escape. While being essential for a proper immune system functioning, glutamine and levels were shown to be lower, while adenosine was shown to be higher, within the tumor microenvironment, thereby conferring a peculiar immunometabolic profile to this model. B. PDL1 blockade triggers a decrease of tumoral adenosine production. Mice were challenged with MCA205 tumors, treated or not with anti-PDL1, and then processed for intratumoral microdialysis. Adenosine levels were measured by LC/MS. Anti-PDL1 induced a limitation of the tumoral adenosine level thus arguing for an important role of the adenosine axis in the control of the anti-tumor immune response.
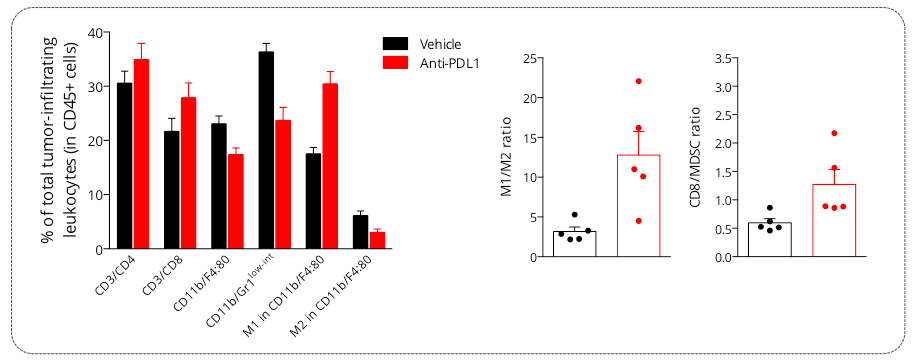
Figure 4: Analysis of PDL1 blockade mechanism of action on the MCA205 tumor-infiltrating leukocytes populations. Flow cytometry analysis of MCA205 tumors shows infiltration by both lymphocytic (CD4, CD8) and myeloid cells including the immunosuppressive MDSC population (CD11b/Gr1low-int) and macrophages (M1 and M2 subsets). Upon anti-PDL1 treatment, investigation of tumor-infiltrating leukocytic cell subsets showed an increase in CD8 cytotoxic lymphocyte proportion as well as decrease of MDSC cell infiltration. In addition, while vehicle tumors were shown to be infiltrated by M2 macrophages, which helps conferring an immunosuppressive microenvironment, PDL1 blockade was demonstrated to reprogram this macrophage profile by increasing the M1 and decreasing the M2 subset proportions, thereby suggesting an effect on macrophage polarization and proliferation.
Case study 2 – Orthotopically-inoculated GL261 glioma mouse tumor model
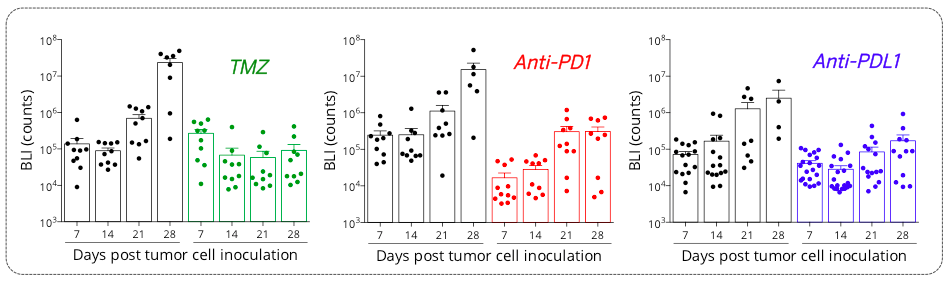
Figure 5: Orthotopic syngeneic GL261 glioblastoma model is responsive to TMZ and PD1/PDL1 axis blockade. Mice were OT inoculated with GL261-Luc2 glioma cells and then treated with either Vehicles, temozolomide (TMZ), anti-PD1 or anti-PDL1 antibodies. Bioluminescence imaging was performed once a week starting from day7 post-tumor inoculation, on days 7, 14, 21, and 28 post-tumor cell inoculation.
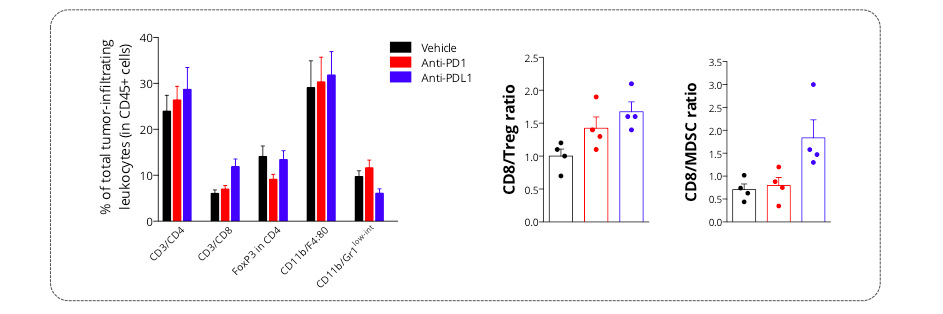
Figure 6: Intracranial glioma tumor-bearing model is characterized by an immunoprivileged microenvironment. Flow cytometry analysis of GL261 tumors shows infiltration by both lymphocytic (CD4, CD8, and Treg (FoxP3+/CD4+) subsets) and myeloid cells including the immunosuppressive MDSC population (CD11b/Gr1low-int). Those key immune cell subsets were shown to be differentially modulated upon treatment with either anti-PD1 or anti-PDL1 antibody – Treg cell population being mainly decreased upon PD1 blockade, while anti-PDL1 antibodies was shown to both increase CD8 and decrease MDSC cell infiltration.