Microdialysis is to date the only in vivo sampling technique used for continuous therapeutic drug or metabolite/analyte monitoring and assessment of surrogate markers for response and toxicity, in the extracellular fluid (ECF) of tissues. The use of this non/minimally invasive microdialysis-based methodology in tumors is relatively new and is becoming more and more important, to evaluate, for instance, tumor ECF disposition of anti-cancer agents and factors affecting delivery and removal of anti-cancer agents, gene therapy, angiogenesis inhibitors… Furthermore, intratumoral microdialysis is becoming an invaluable tool for the assessment of physiological and pathophysiological role of metabolic/signaling pathways involved in the tumor metabolism and tumor immune escape. Technically, intratumoral microdialysis allows for providing comprehensive means to serially sample the tumor ECF – with minimal tissue damage or fluid balance alteration – from which a concentration can be determined versus time-profile. In addition, a single microdialysis probe can simultaneously allow for sampling several analytes of interest, thereby enabling the evaluation of pharmacokinetic and pharmacodynamic changes.
A dedicated platform is thus available to run intratumoral microdialysis in valuable in vivo tumor models, and combined with suitable a detection method, for the evaluation of therapeutic anti-cancer agents and/or surrogate markers in response to treatments.
- Microdialysis allows measuring the release and metabolism of molecules / markers of interest diffusing in the ECF of the tumor.
- Appropriate to assess physiological and pathophysiological role of metabolic and signaling pathways involved in the tumor metabolism.
- Well-suited for PK and PD monitoring of drug and/or intended analyte levels, when combined to a suitable detection method.
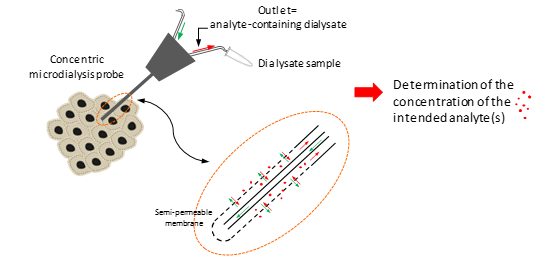
Schematic illustration of intra-tumoral microdialysis technique for tumor extracellular fluid sampling and analysis.
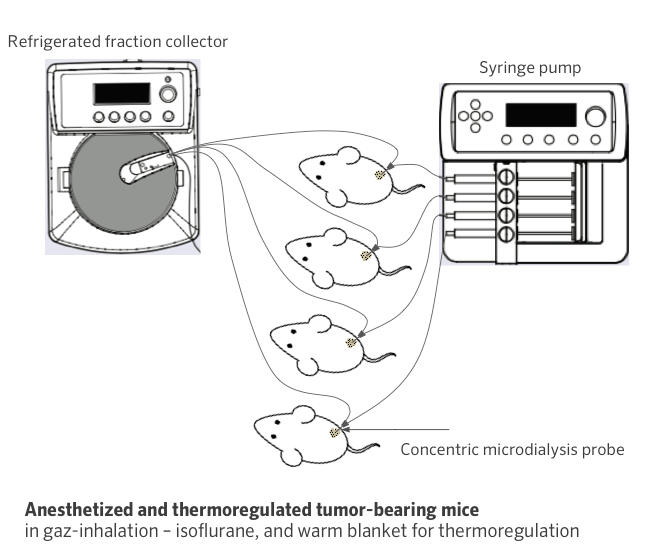
Schematic illustration of in-house microdialysis platform
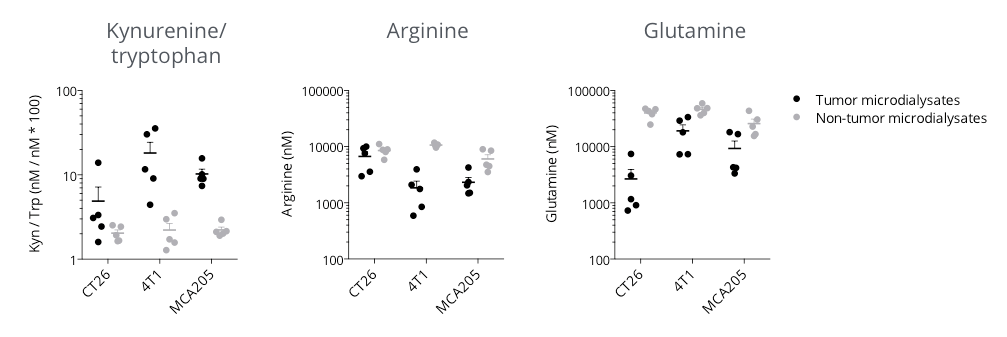
Microdialysis-based immunometabolic pathway profiling in CT26 colon, 4T1 breast, and MCA205 sarcoma tumor-bearing mouse models.
Mice were appropriately implanted with respective tumor cells and then processed for intratumoral vs. non-tumoral microdialysis sampling – when tumors reached ~200mm3, for the determination of kynurenine, tryptophan, arginine and glutamine levels by LC/MS. The three tumor models display differential features for kynurenine/tryptophan, arginine, and glutamine pathways – known to be involved in the immune escape. While being essential for a proper immune system functioning, tryptophan (kynurenine/tryptophan ratio), arginine, and glutamine levels were shown to be lower within the tumor microenvironment with respect to non-tumor compartments, thereby conferring a specific immunometabolic profile to each tumor model.
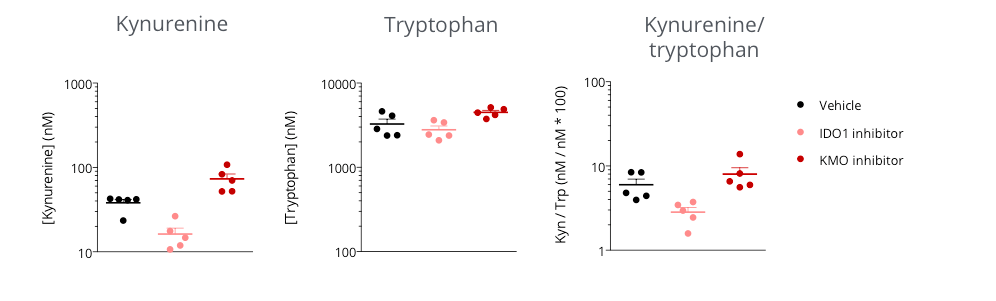
Assessment of kynurenine/tryptophan pathway modulation in the subcutaneously-implanted MCA205 sarcoma tumor-bearing mouse model through microdialysis-based intratumoral kynurenine level quantification.
Mice were appropriately challenged with MCA205 tumors, exposed to either reference indoleamine 2,3-dioxygenase 1 (IDO) inhibitor or kynurenine-3-monooxygenase (KMO) inhibitor, and then processed for intratumoral microdialysis. Kynurenine and Tryptophan levels were measured by LC/MS. While IDO inhibition was shown to decrease the intratumoral level of kynurenine and thus the kynurenine/tryptophan ratio, protecting from kynurenine degradation under KMO inhibitor treatment resulted in higher kynurenine levels, compared to vehicle control.