Syngeneic tumor models were becoming invaluable for preclinical development and evaluation of immuno-based therapies. Nevertheless, the immunometabolic features of these tumors models, which may likely underlie responsiveness to immunotherapies, are still not well understood and become thus primordial to be defined for tumor model characterization and selection of the appropriate ones, for target validation, drug development, and assessment of the efficacy potential of drug candidates.
Across an array of our syngeneic tumor models, we used intratumoral microdialysis for tumor extracellular fluid sampling and immunometabolic profiling for three pathways whose activation is known to be involved in tumor immune escape and resistance to gold standard immunotherapies. As illustrated below, intratumoral microdialysis, combined with complementary analytical methods, is revealed as a relevant approach for the determination of the tumor immunometabolic features – through quantification of respective metabolites as surrogate markers, not only in basal conditions but also upon treatments expected to modulate those pathways.
Using this powerful technique and revealing specific tumor immunometabolic differences, which may be supporting differential responsiveness profiles, would enable appropriate model selection to interrogate the efficacy of novel immunotherapies and drug candidates targeting specific immunometabolic pathways, alone and in combination with immune checkpoint inhibitors.
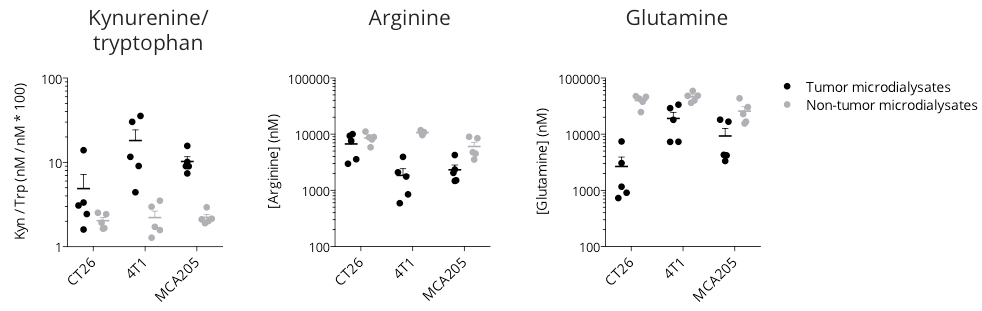
Figure 1: Microdialysis-based immunometabolic pathway profiling in CT26 colon, 4T1 breast, and MCA205 sarcoma tumor-bearing mouse models. Mice were appropriately implanted with respective tumor cells and then processed for intratumoral vs. non-tumoral microdialysis sampling – when tumors reached ~200mm3, for the determination of kynurenine, tryptophan, arginine and glutamine levels by LC/MS.
The three tumor models display differential features for kynurenine/tryptophan, arginine, and glutamine pathways – known to be involved in the immune escape. While being essential for a proper immune system functioning, tryptophan (kynurenine/tryptophan ratio), arginine, and glutamine levels were shown to be lower within the tumor microenvironment with respect to non-tumor compartments, thereby conferring a specific immunometabolic profile to each tumor model.
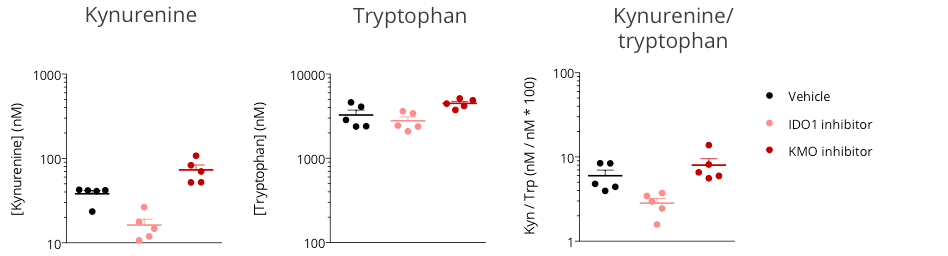
Figure 2: Assessment of kynurenine/tryptophan pathway modulation in the subcutaneously-implanted MCA205 sarcoma tumor-bearing mouse model through microdialysis-based intratumoral kynurenine level quantification. Mice were appropriately challenged with MCA205 tumors, exposed to either reference indoleamine 2,3-dioxygenase 1 (IDO) inhibitor or kynurenine-3-monooxygenase (KMO) inhibitor, and then processed for intratumoral microdialysis. kynurenine and tryptophan levels were measured by LC/MS.
While IDO inhibition was shown to decrease the intratumoral level of kynurenine and thus the kynurenine/tryptophan ratio, protecting from kynurenine degradation under KMO inhibitor treatment resulted in higher kynurenine levels, compared to vehicle control.