Undifferentiated pleomorphic sarcoma (UPS) is one of the most common—and most aggressive—subtypes of soft tissue sarcoma (STS). Despite optimal treatment in localized stages, nearly half of patients ultimately develop metastatic disease, which drives poor outcomes.
To better understand the mechanisms underlying UPS metastasis, a team led by Prof. Antoine Italiano (Institut Bergonié, University of Bordeaux, INSERM U1312 BRIC) performed an integrated multi-omics analysis of paired primary and metastatic UPS samples, followed by functional validation in relevant patient-derived models.
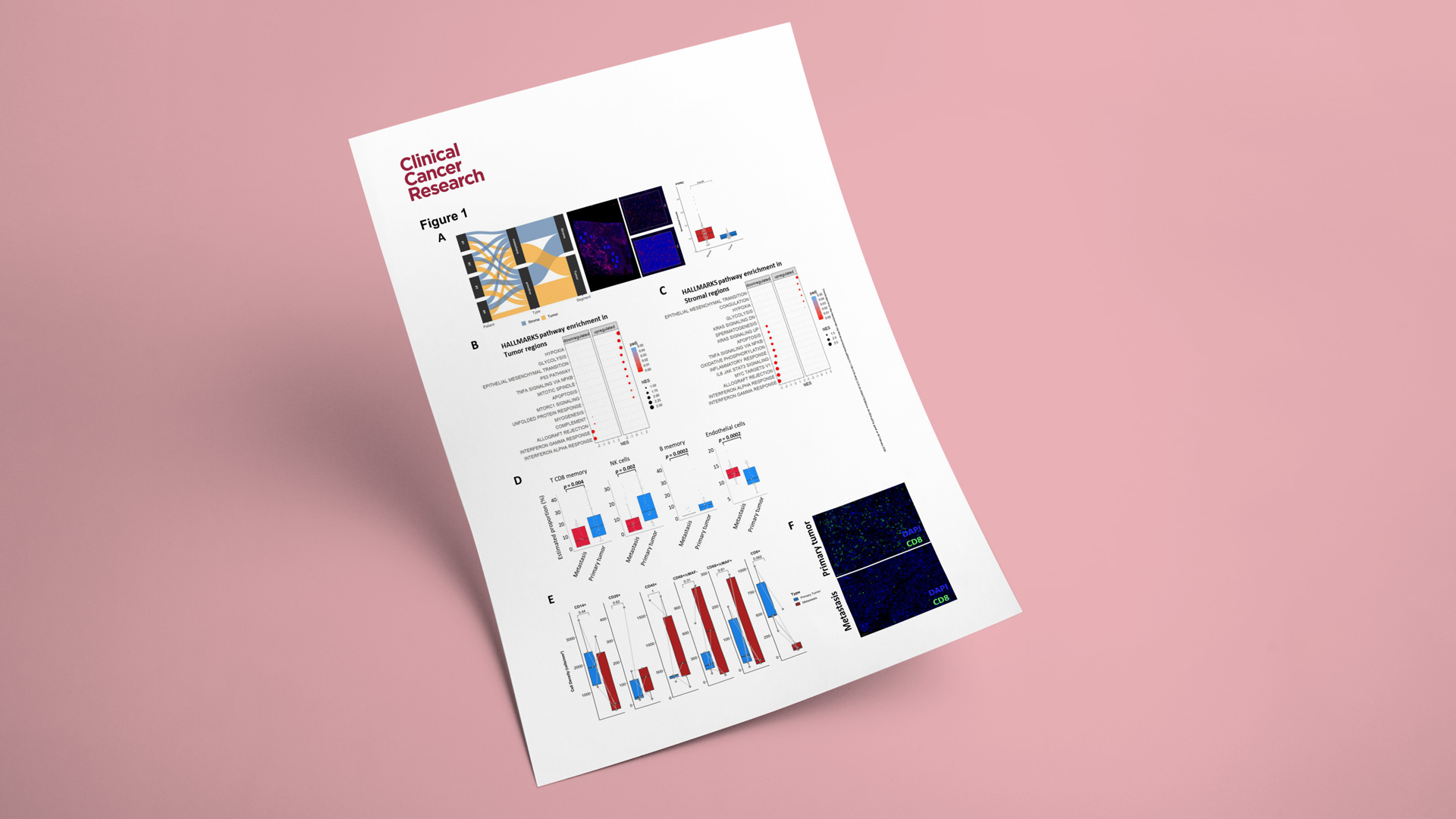
As part of this work, Explicyte performed spatial transcriptomic profiling using the GeoMx Digital Spatial Profiler (Whole Transcriptome Atlas; >18K genes). The spatial analyses were conducted on 3 paired primary tumors and matched metastases enabling a direct comparison of tumor and microenvironmental programs across disease stages.
Key observations included:
To further support these findings, we confirmed a reduction in CD8+ T-cell infiltration in metastases using a multiplex immunohistofluorescence (mIHF) panel in five paired primary/metastatic cases.
In parallel, the team analyzed paired primary and metastatic tumor samples from 13 patients using bulk RNA-seq to capture global transcriptomic changes associated with metastatic progression. This analysis confirmed an enrichment of metastasis-associated pathways and identified 690 genes significantly altered between primary tumors and metastases. Among the top candidates, ADORA2B emerged as particularly compelling because:
Using public and third-party sarcoma datasets, the team further showed that ADORA2B is overexpressed in UPS and is associated with features of an immune-suppressive microenvironment in metastatic UPS—supporting ADORA2B as a promising therapeutic target in this indication.
To test whether ADORA2B plays a causal role in UPS aggressiveness, the authors generated ADORA2B knockout models using CRISPR-Cas9 in two patient-derived UPS cell lines.
The in vivo impact was then evaluated in Rag2-/- γc-/- mice:
Finally, the team evaluated a dual ADORA2A/ADORA2B inhibitor, M1069 (EMD Serono), currently assessed in early-phase trials. In vitro, M1069 reduced proliferation and invasion in UPS models, providing pharmacological support for the genetic findings.
Overall, this work identifies ADORA2B as a critical regulator of primary tumor growth and metastatic dissemination in UPS. It also highlights the therapeutic promise of targeting the adenosine axis, and specifically ADORA2B, as a strategy to disrupt metastatic progression and improve outcomes in this rare and aggressive cancer.
This research received funding from the Agence Nationale de la Recherche (“France 2030” / ANR 21 RHUS 0010).
To better understand the mechanisms underlying UPS metastasis, a team led by Prof. Antoine Italiano (Institut Bergonié, University of Bordeaux, INSERM U1312 BRIC) performed an integrated multi-omics analysis of paired primary and metastatic UPS samples, followed by functional validation in relevant patient-derived models.
Spatial biology (Explicyte)
As part of this work, Explicyte performed spatial transcriptomic profiling using the GeoMx Digital Spatial Profiler (Whole Transcriptome Atlas; >18K genes). The spatial analyses were conducted on 3 paired primary tumors and matched metastases enabling a direct comparison of tumor and microenvironmental programs across disease stages.
Key observations included:
- Upregulation of hypoxia, glycolysis, and EMT-related programs in metastases, consistent with a more aggressive, metastasis-associated biology.
- Enrichment of endothelial-cell signatures in metastatic samples, suggesting increased angiogenesis compared with primary tumors.
- Higher immune infiltration in primary tumors (including CD8+ T cells, NK cells, and memory B cells), supporting the notion of a more immune-suppressive / immune-excluded state in metastatic lesions.
To further support these findings, we confirmed a reduction in CD8+ T-cell infiltration in metastases using a multiplex immunohistofluorescence (mIHF) panel in five paired primary/metastatic cases.
Bulk RNAseq
In parallel, the team analyzed paired primary and metastatic tumor samples from 13 patients using bulk RNA-seq to capture global transcriptomic changes associated with metastatic progression. This analysis confirmed an enrichment of metastasis-associated pathways and identified 690 genes significantly altered between primary tumors and metastases. Among the top candidates, ADORA2B emerged as particularly compelling because:
- ADORA2B encodes a G protein–coupled adenosine receptor (A2B) implicated in metastatic progression in several epithelial cancers,
- ADORA2B is druggable, with inhibitors already being evaluated in clinical development programs.
Using public and third-party sarcoma datasets, the team further showed that ADORA2B is overexpressed in UPS and is associated with features of an immune-suppressive microenvironment in metastatic UPS—supporting ADORA2B as a promising therapeutic target in this indication.
Functional validation
To test whether ADORA2B plays a causal role in UPS aggressiveness, the authors generated ADORA2B knockout models using CRISPR-Cas9 in two patient-derived UPS cell lines.
- Transcriptomic profiling of ADORA2B-knockout cells confirmed downregulation of key pathways involved in metastatic biology.
- Functional assays demonstrated that ADORA2B loss reduces UPS cell proliferation, migration, and invasion, supporting a direct role in tumor aggressiveness.
The in vivo impact was then evaluated in Rag2-/- γc-/- mice:
- In an orthotopic model, tumors derived from ADORA2B-knockout cells showed markedly impaired growth, leading to significantly smaller, low-proliferating tumors compared with controls.
- In a forced metastasis model (tail-vein injection), ADORA2B-knockout cells produced substantially fewer metastases, translating into a striking survival benefit (100% survival in the knockout arm vs 0% in controls in this experimental setting).
Finally, the team evaluated a dual ADORA2A/ADORA2B inhibitor, M1069 (EMD Serono), currently assessed in early-phase trials. In vitro, M1069 reduced proliferation and invasion in UPS models, providing pharmacological support for the genetic findings.
Impact
Overall, this work identifies ADORA2B as a critical regulator of primary tumor growth and metastatic dissemination in UPS. It also highlights the therapeutic promise of targeting the adenosine axis, and specifically ADORA2B, as a strategy to disrupt metastatic progression and improve outcomes in this rare and aggressive cancer.
Read the paper
This research received funding from the Agence Nationale de la Recherche (“France 2030” / ANR 21 RHUS 0010).