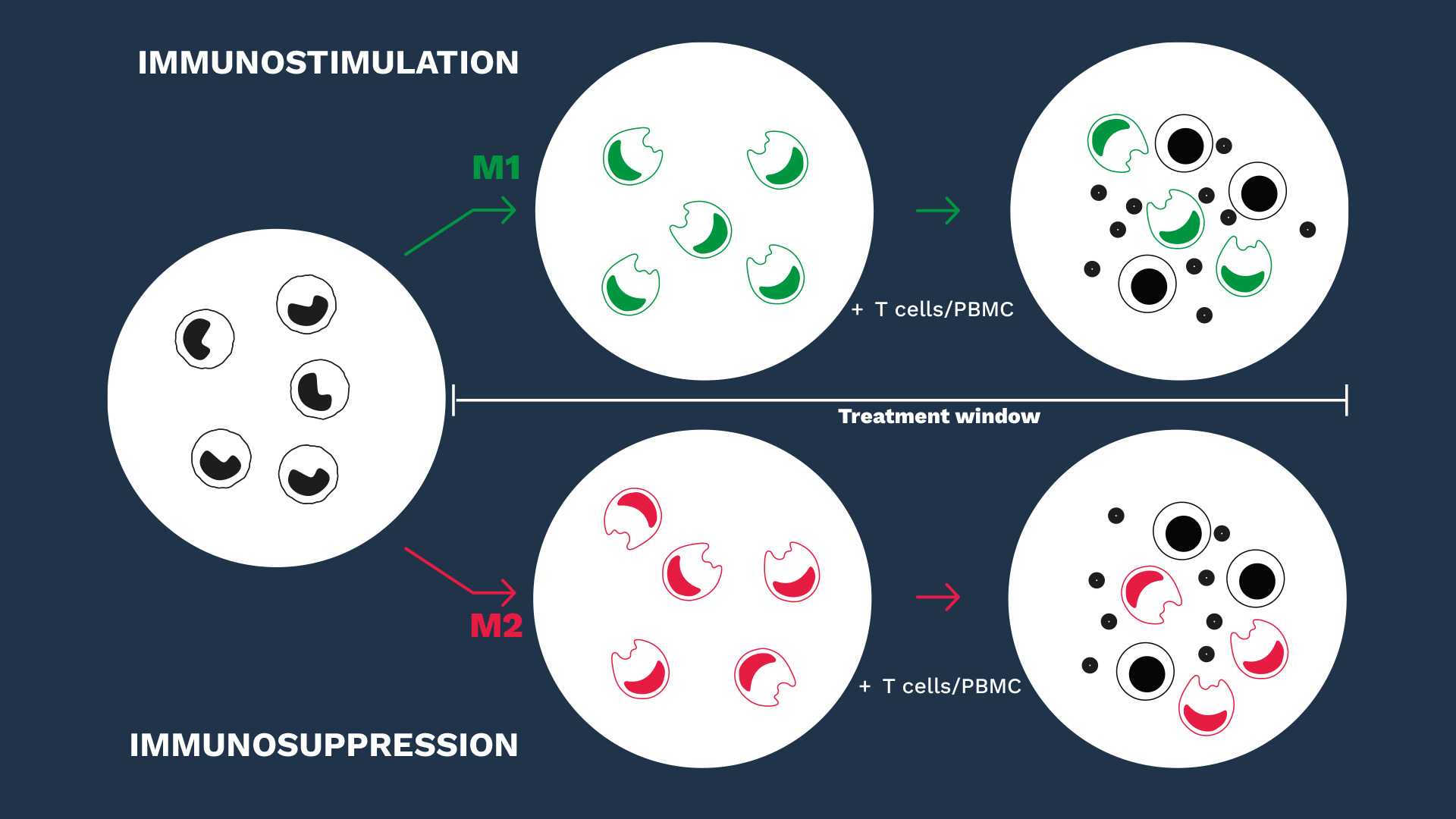
While macrophages play a central role in innate immune inflammatory mechanisms and in priming adaptive responses, they exhibit phenotypically and functionally distinct subsets, influenced by their environment and local stimuli.
In cancer and as a key component of the tumor microenvironment, they strongly regulate immunity and differentially influence tumorigenesis, depending on surrounding cues.
Unlike M1 macrophages that promote proinflammatory responses, M2 macrophages display immunoregulatory functions and trigger inflammation resolution and immunosuppression, thereby mediating tumor promotion.
Behind these opposing subsets, macrophages exhibit a level of plasticity and are themselves capable of mutually regulating each other along a sliding scale between pro- and anti-inflammatory functions.
Therefore, modifying or interfering with macrophage polarization or their switch from an M2 to an M1 phenotype remains an attractive strategy to potentiate the effector immune response. In this context, in vitro macrophage-based assays are relevant to assess immunotherapeutics and question their mechanisms of action.
While mastering the key specifications of our macrophage-based models and their subtleties, our scientific and experimental expertise governs the relevance of our macrophage assays in evaluating the efficacy of immunotherapeutics either on the stimulatory M1 capacities or, especially, in relieving the suppressive functions of M2 macrophages.
Discover our macrophage assays
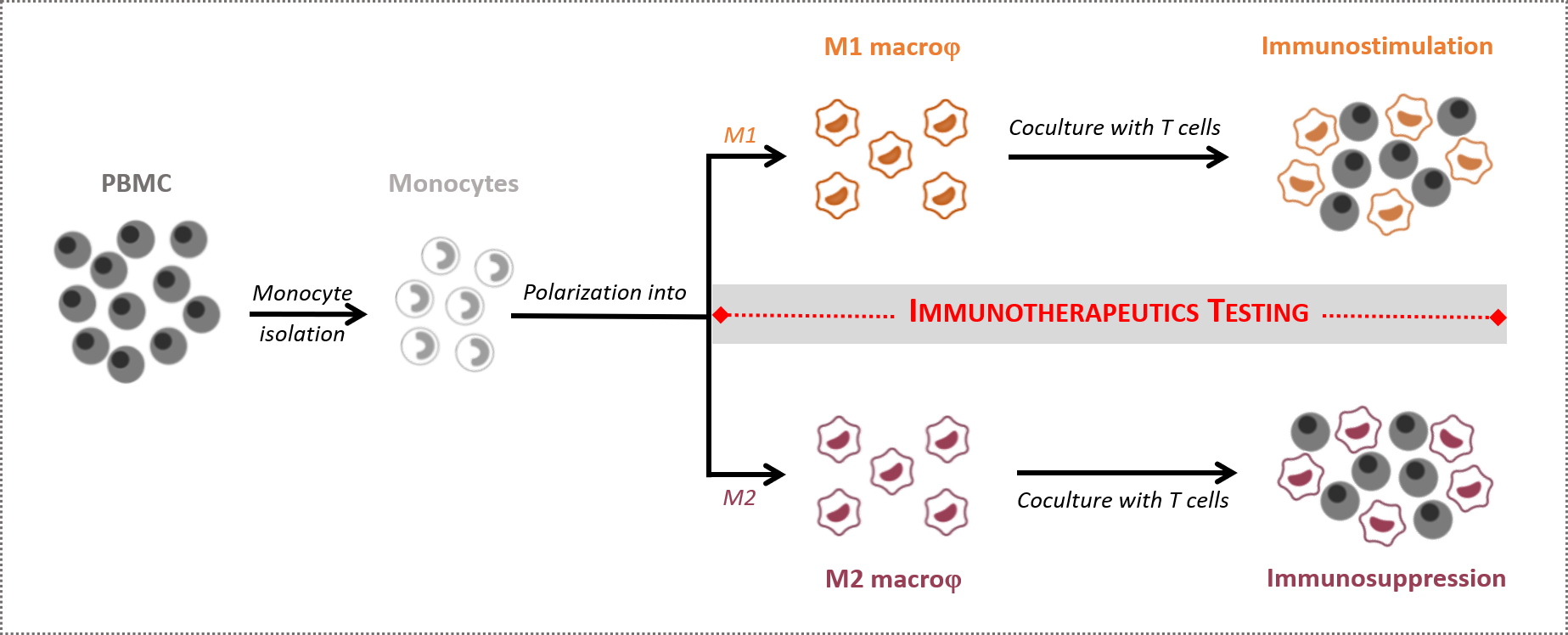
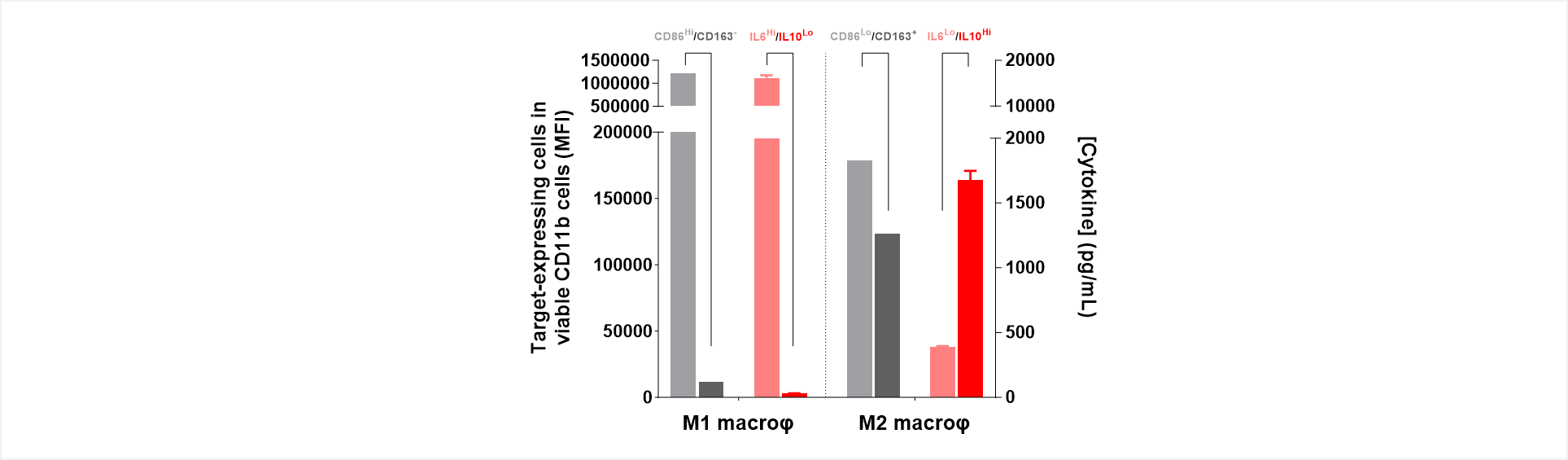
Figure 1: Mirrored phenotypes of marker expression and cytokinic profile feature functionally opposite M1/M2 macrophage subsets.
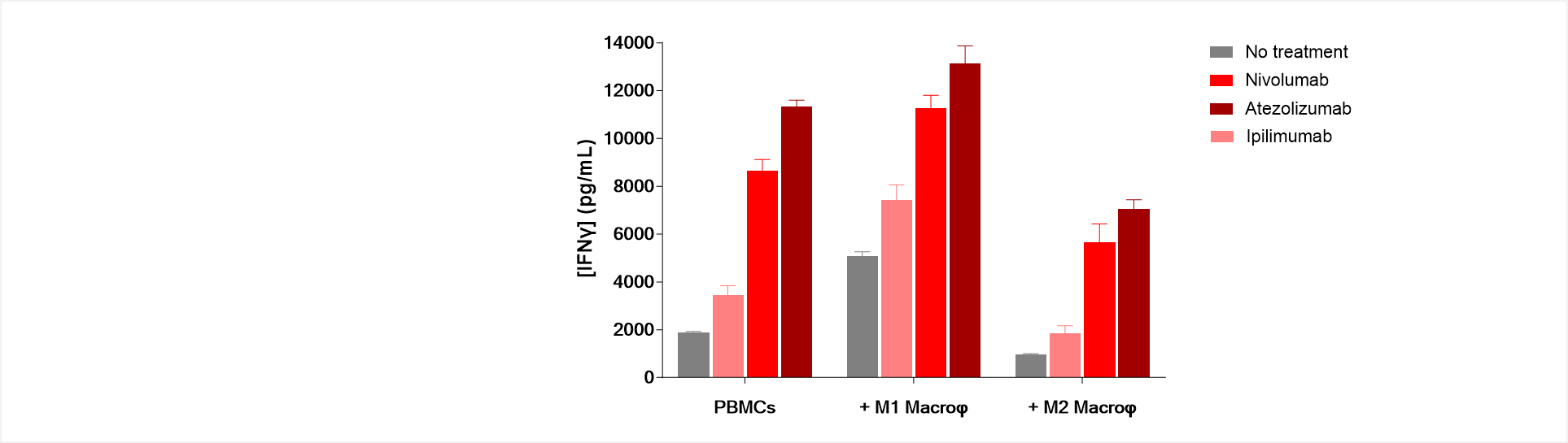
Figure 2: Immune checkpoint blockade antagonizes M2-mediated immunosuppression while promoting M1-mediated immune response stimulation.
Unlike M1 macrophages, which stimulate T cell response thereby inducing an increase in IFNγ production in anti-CD3-activated PBMC/M1 co-culture, M2 macrophages exhibit a suppressive activity of T cell activity shown through IFNγ level decrease.
M2 suppression function is known to be exerted via multiple mechanisms including PD1/PDL1 axis engagement, but also CTLA4/CD80 and CD86. Interestingly, treatments of PBMC/M2 co-cultures with either Nivolumab, Atezolizumab, or even Ipilimumab result in a reversal of the T cell immunosuppression compared to untreated PBMC/M2 co-cultures. On the contrary, these immune checkpoint inhibitors further promote the M1-mediated T cell response stimulation.