As in the previous shuttle session, Explicyte will plan a new shuttle on the CT26 tumor-bearing mouse model, which will be held in July.
In this shuttle, our well-characterized syngeneic CT26 colon tumor-bearing mouse model will be used through standardized study protocols, for the evaluation of potential anti-tumor benefits from drug combinations with reference immune checkpoint inhibitors or chemotherapies.
Interestingly, this new shuttle session offers you the opportunity to test your compound in a cost-saving manner since only experimental test groups will be at the Sponsor’s expense, while vehicle- and reference-treated groups will be supported by Explicyte. Therefore, in order to take part of this shuttle session, contact us to book a place & test your candidate compound.
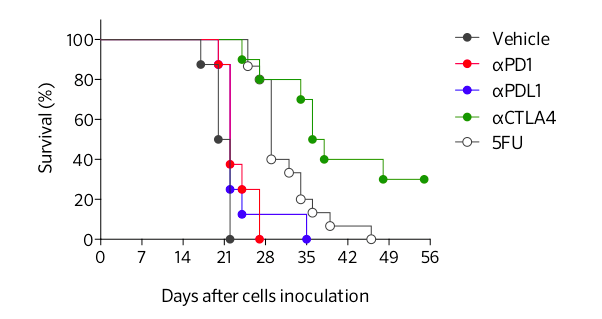
Differential responses of CT26 tumor-bearing mouse model to immune checkpoint inhibitors and to 5-Fluorouracil (5FU) chemotherapy. Mice are challenged with CT26 tumor cells and exposed to either anti-CTLA4, anti-PD1 or anti-PDL1 antibodies, or to 5FU treatment. Contrarily to the strong response to anti-CTLA4 antibody, CT26 tumor is partially responsive to PD1/PDL-1 blockade. A partial response is also observed upon 5FU treatment.
Shuttle for efficacy assessment on 6-week experimental session
- Model / Strain: CT26 colon tumor-bearing model / Balb/c mice
- Readouts: Tumor growth / Body weight / Survival
- Standard reference: PD1, PDL1, or CTLA4 monoclonal antibody, or 5FU treatment
- Group size: At least 10 mice per group (with a minimum of 2 groups)