We will be attending the AACR Virtual Annual Meeting, from June 22 to 24, 2020 to present our posters featuring our capacities / platforms that support drug discovery in Immuno-Oncology.
Deciphering anti-PDL1 effect in a syngeneic mouse model of sarcoma
Presenter/Authors
Imane Nafia, Jean-Philippe Guegan, Assia Chaibi, Doriane Bortolotto, Loïc Cerf, Manon Clemenceau, Antoine Italiano, Alban Bessede. Explicyte, Bordeaux, France, Institut Bergonié, Bordeaux, France
Abstract
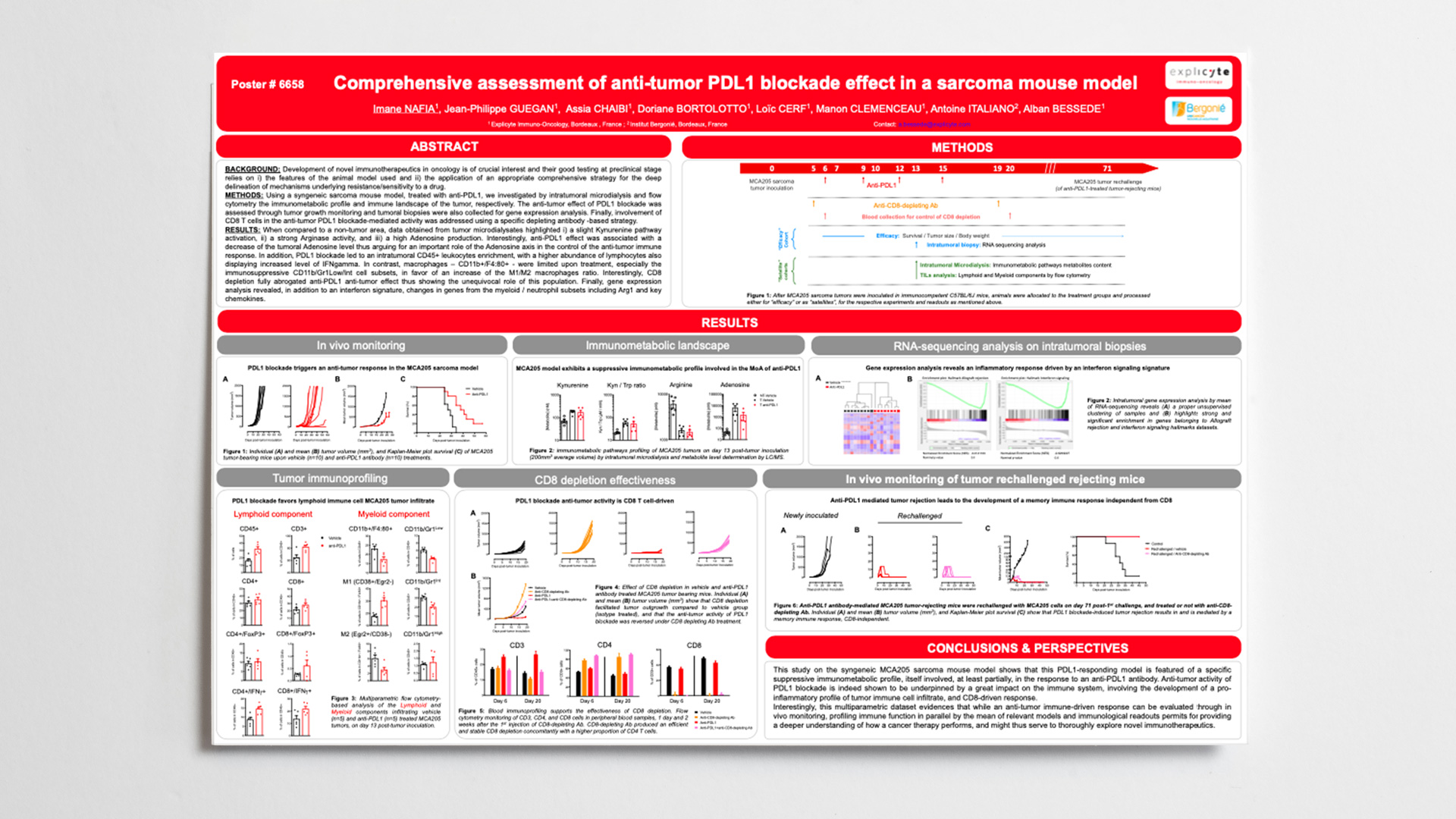
Background: In the context of novel immuno-oncology therapeutics development at preclinical stage, it’s crucial to understand the primary features of the in-vivo model to be used and to delineate mechanisms underlying response to a drug candidate. While PDL1 blockade figures as a gold standard immunotherapy its exact mechanism of action in a mouse model of sarcoma remains to be elucidated.
Methods: Using a preclinical syngeneic mouse model of sarcoma based on the inoculation of MCA205 cell line we investigated anti-tumor effect of anti-PDL1 antibody through tumor growth and survival assessment. Using the same experimental setting, and by mean of intratumoral microdialysis, immune-related metabolic pathways at both tumor and contralateral sites were assessed. Tumor samples were also collected for gene expression analysis by RNA sequencing. Also, immune landscape of the tumor was addressed by both flow cytometry analysis and by multiparametric immunohistofluorescence and image analysis. Finally, participation of CD8 T cells was investigated using a specific depleting antibody-based strategy.
Results: As expected, administration of anti-PDL1 antibody led to a strong anti-tumor response. With respect to the immunometabolism features of this model, it displays within the tumor – and compared to the non-tumor bearing area – i) a slight Kynurenine pathway activation, ii) a strong Arginase activity, and iii) a high Adenosine production. Interestingly, anti-PDL1 effect was associated with a limitation of Adenosine level within the tumor thus arguing for an important role of the Adenosine axis in the control of the anti-tumor immune response. Along with this effect, PDL1 blockade led to an intratumoral enrichment of leukocytes (CD45+), with a higher abundance of lymphocytes also displaying increased level of IFNgamma. In contrast, macrophages – delineated as CD11b+/F4:80+ – were limited upon treatment, especially the immunosuppressive CD11b/Gr1Low/Int cell subsets while concomitantly promoting a M1/M2 macrophages ratio. Along with these data, RNAseq analysis revealed an enrichment in genes from immune-related datasets including interferon signaling. Interestingly, CD8 depletion fully abrogated anti-PDL1 anti-tumor effect thus confirming the critical role of this population.
Conclusions: Altogether, this new multiparametric dataset collected on a preclinical model of sarcoma allows the highlighting of specific intrinsic features of the model, and certainly provides a deeper understanding of the mechanism of action of an effective and approved drug. Such a comprehensive assessment-based strategy can therefore serve as a basis for further exploration of novel candidates.
To learn more about our in vivo services for the evaluation of cancer immunotherapies