Digital pathology for tumor immune infiltrate analysis and patient selection
To assess whether or not the tumor immune contexture was predictive of response to the Avelumab-Regorafenib combination regimen, Explicyte developed and ran, on baseline FFPE samples, a multiplexed immunohistofluorescence panel consisting of CD8, PD1, PDL1, CD163, IDO1, and PanKeratin markers, in addition to Dapi (Discovery XT, Roche Diagnostics).
While the development of immune checkpoint inhibitors (ICI) has dramatically changed the therapeutic armamentarium in several cancers, MicroSatellite Stable (MSS) colorectal cancer (CRC) remains insensitive to cancer immunotherapy and clinical assessment of novel combination strategies is thus needed. Given the emerging role of tumor vasculature in the response to cancer immunotherapy [1], it was hypothesized that targeting one of its key axis, i.e. VEGF-A/VEGFR, in combination with anti–programmed cell death 1 (PD1)/anti–programmed cell death ligand 1 (PDL1) antibodies may be associated with clinical benefit in patients with MSS metastatic colorectal cancer having failed previous standard chemotherapy regimens.
REGOMUNE, a multicenter, single-arm, basket trial, sponsored by Institut Bergonié (Bordeaux, FRANCE), aimed at evaluating the benefit of Avelumab – an anti-PDL1 antibody – in combination with Regorafenib, a VEGF and CSF1 receptors tyrosine kinase inhibitor in patients with advanced solid tumors. Cousin et al. [2] reported the results from the MSS colorectal cancer cohort. With a median progression-free survival (PFS) and a median overall survival (OS) of 3.6 and 10.8 months, respectively, these results compare favorably with i) the median PFS of 1.9 months and ii) median OS of 6.4 months, observed with Regorafenib used as a single agent in the pivotal study which led to approval [3].
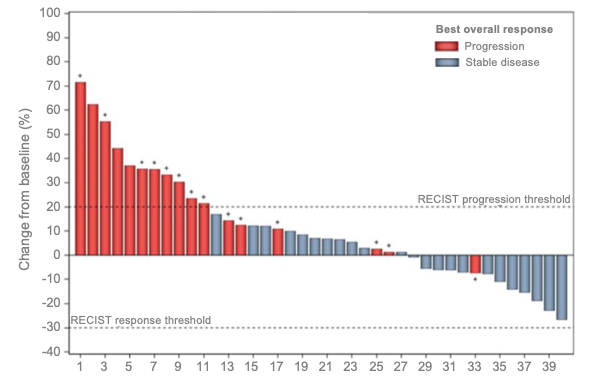
Figure 1: Waterfall plot of best overall response in MSS CRC patients treated with Regorafenib plus Avelumab (n=40, response based on central review assessment according to RECIST 1.1).
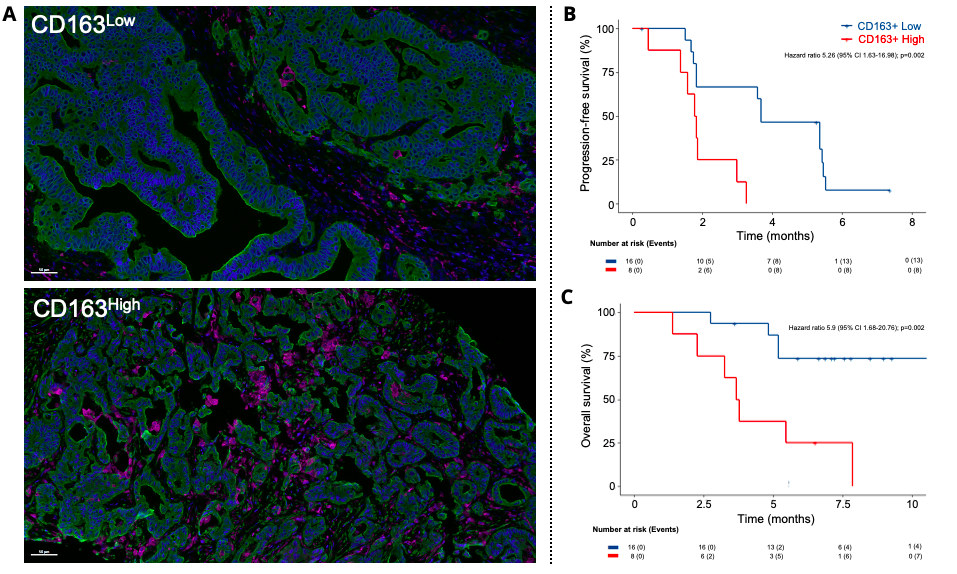
Figure 2: Density of CD163+ macrophages within the tumor is predictive of outcome in cancer patients treated with Regorafenib and Avelumab. A. The presence of CD163+ macrophages within the tumor was calculated in all tumor samples collected before treatment initiation and was based on a multiplexed immunohistofluorescence staining combining panKeratin (PanCK, green) and CD163 (Purple) markers. Slide digitization and image analysis were then performed to calculate CD163+ cells density within the tumor lesion. Image on top illustrates a patient displaying a low level of CD163 infiltration while the image at the bottom is representative of a case with high CD163+ cells density. Kaplan-Meier curves of B. progression-free survival and C. overall survival in a cohort of 24 microsatellite stable CRC patients treated with Regorafenib and Avelumab according to the density of intratumoral CD163+ macrophages.
Learn more about our services in digital pathology
[1] Fukumura et al., Nat Rev Clin Oncol., 2018 May 15