Explicyte is planning a new in vivo shuttle session by early March, thereby providing an opportunity to cost-effectively assess your drug candidates in our well-suited immunocompetent syngeneic subcutaneously-implanted tumor models, over studies where a significant cost part will be supported by ourselves.
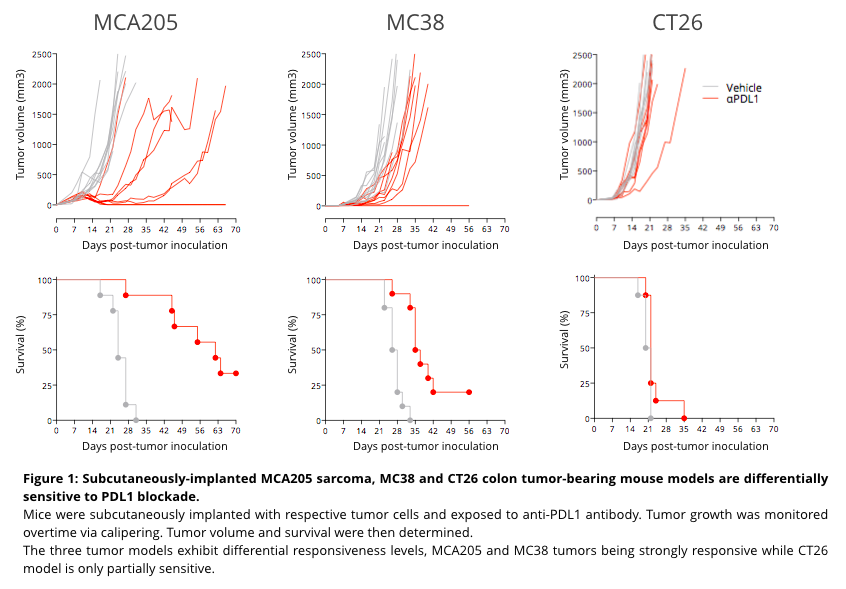
Our well-characterized syngeneic MCA205 sarcoma, MC38 and CT26 colon tumor-bearing mouse models, differentially sensitive to PDL1 blockade, will be used in this shuttle through standardized study protocols, for the evaluation of potential anti-tumor benefits from candidate compounds alone and in combination with our reference anti-PDL1 antibody.
As cost-effective studies, only experimental test groups will be at the Sponsor’s expense, while vehicle- and reference-treated groups will be supported by Explicyte.
Shuttle for efficacy assessment on 6-week experimental session
- Model / Strain: According to our Sponsor’s requirements
- Readouts: Tumor growth / Body weight / Survival
- Standard reference: 𝛂PDL1 monoclonal antibody – to be discussed with the Sponsor
- Group size: At least 10 mice per group
Optional analyses: Satellite studies for immune response profiling and MoA delineation (flow cytometry, RT-qPCR, immunohistochemistry…). Contact us for further information.
Learn more about our syngeneic tumor models

