The underlying question: Does your compound enhance antigen presentation or trigger clonal expansion of defined tumor-specific antigen-reactive T cells?
- Format: Adapted miniaturized formats for DC cultures, DC-CD8 cocultures and CD8-tumor co-cultures
- Model: Human DC-primed CD8 T cells co-cultured with target tumor cells
- Readouts: Tumor cell apoptosis count by live-cell imaging, and immune cell response via the capture of IFNg release
- Standards: Anti-PD-L1 antibodies (Atezolizumab, Avelumab), anti-PD1 antibodies (Nivolumab, Pembrolizumab)
- On request: Flow cytometry immunophenotyping, assessment of cytokine release, proteomics, transcriptomics
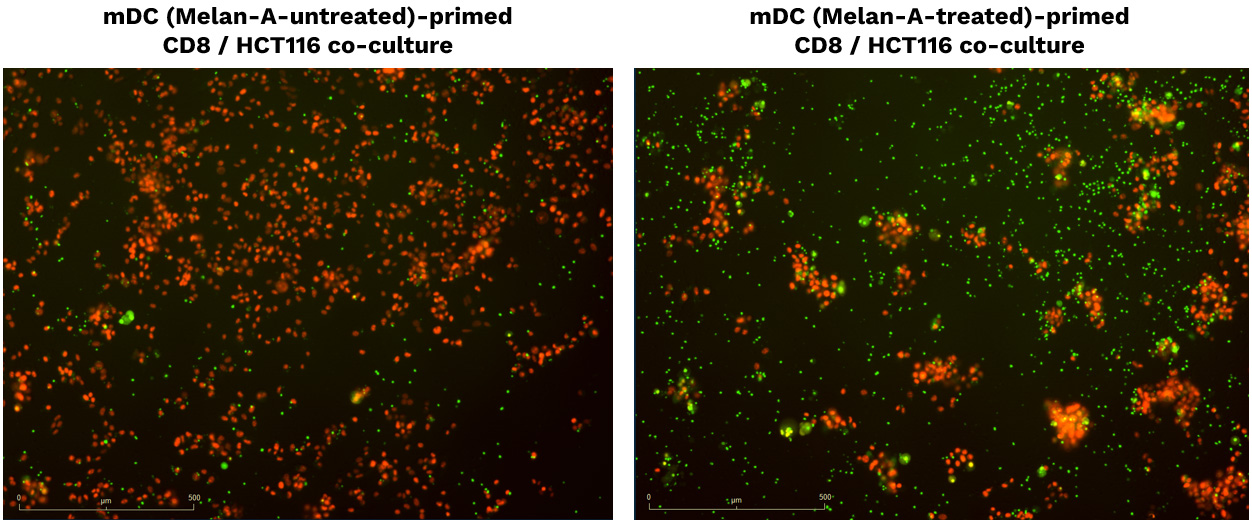
Specific enhanced DC-mediated priming of CD8 T cells induces adaptive T cell-mediated killing towards SK-MEL-5 tumor cells.
SK-MEL-5 (Melan-A+) tumor cells were seeded, and then co-cultured with CD8 T cells primed by either untreated or Melan-A-treated DCs. Tumor cell proliferation (count) & apoptosis were monitored and analyzed as surrogate measures of immune cell-mediated tumor cell killing. Image fields are representative of tumor proliferation / apoptosis at 72h post-coculture.
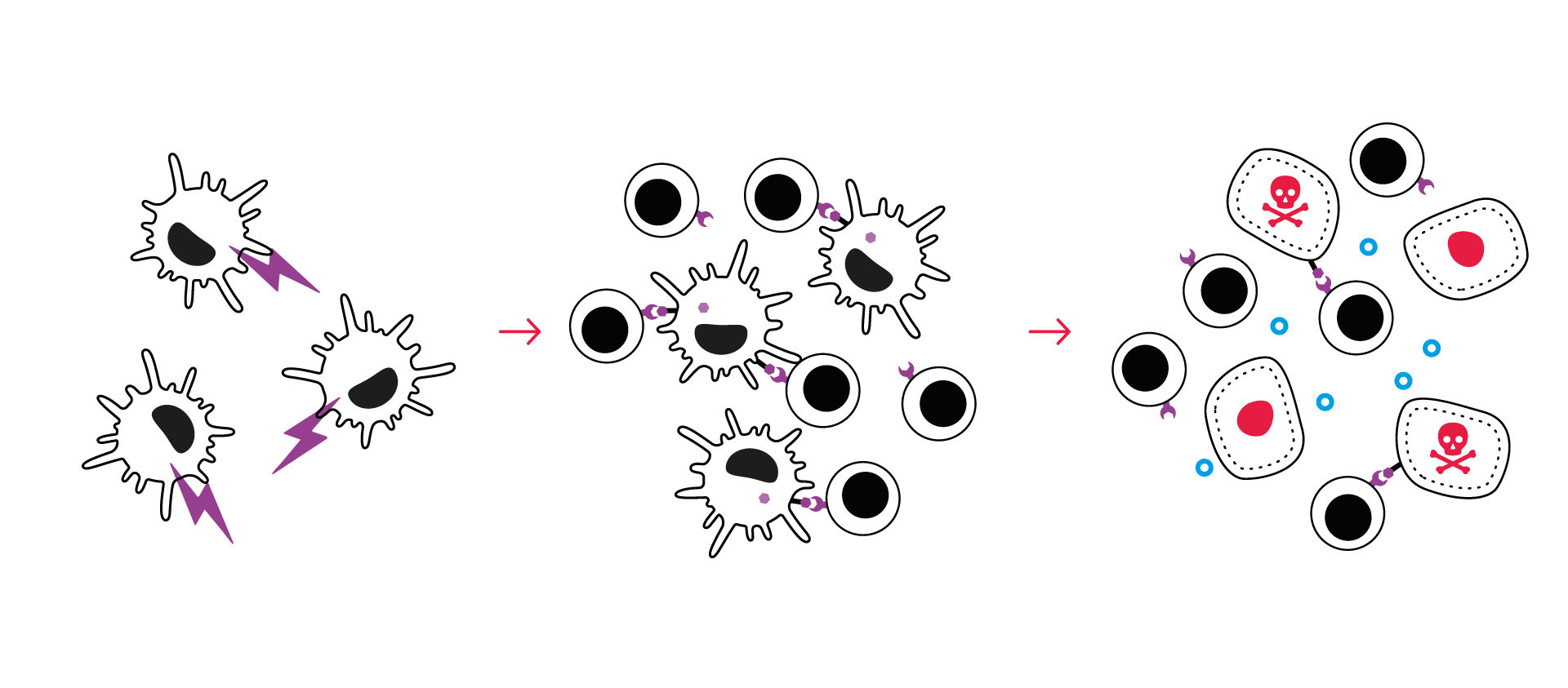
Assay principle
- DC exposure to specific antigen peptides/antigen presentation-enhancing items
- DC-mediated priming of CD8 T cells
- Co-culture of CD8 T cells and target tumor cells
- Parallel capture of tumor & immune components:
- Real-time monitoring of tumor cell count (nuclear fluorescent probe) and tumor cell death (caspase 3/7 fluorescent reagent)
-
- Quantification of IFNg release – surrogate of T cell activation
- Automated image segmentation & analysis
- Additional readouts: immunophenotyping (including differentiation & maturation DC markers), cytokine release (including DC cytokines), gene expression, proteomics, etc
Enhancing DC-mediated tumor antigen presentation triggers priming of specific cytotoxic lymphocytes thereby inducing an adaptive effector T cell-mediated killing response
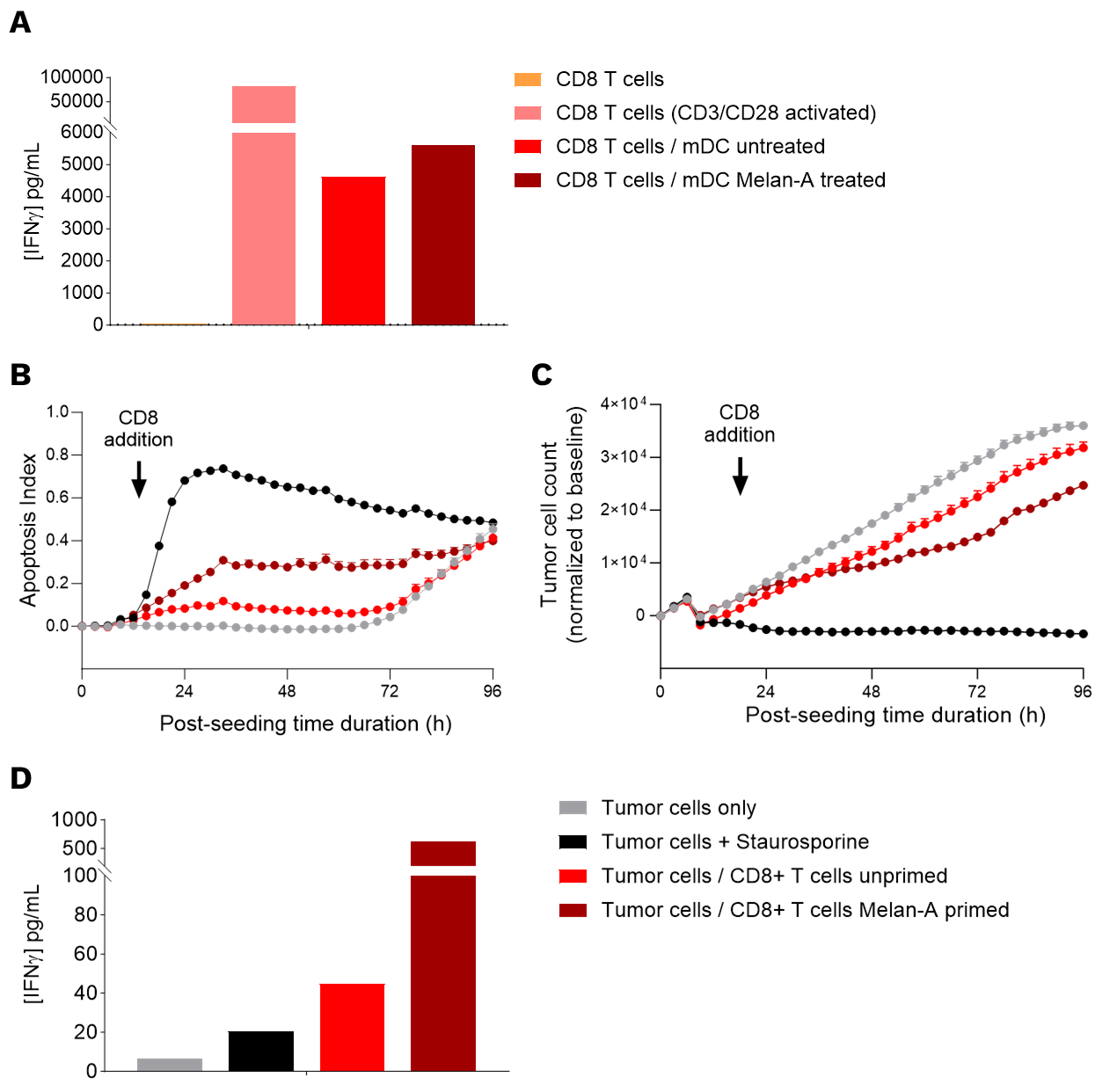
(A) Melan-A antigenic peptide treatment of DCs during their maturation optimizes the priming of CD8 T cells, as shown through the increased IFNγ levels in supernatants of CD8 T cell / mDC co-cultures, compared to the untreated condition.
(B,C,D) Specific enhanced DC-mediated priming of CD8 T cells allows for the induction of an adaptive effector T cell-mediated killing towards SK-MEL-5 tumor cells. (B,C) Real-time monitoring of SK-MEL-5 (Melan-A+) tumor cell killing mediated by CD8+ T cells, either primed or unprimed by Melan-A-treated DCs. Apoptosis rates (B) and tumor cell count (C) were monitored over ~4 days and analyzed. Data were normalized to the baseline and are presented as mean ± SEM. (D) IFNγ levels in supernatants of co-cultures of SK-MEL-5 tumor cells and CD8+ T cells, which were either primed or unprimed by Melan-A-treated DCs.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 100+ in vitro campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- A comprehensive technology platform to decipher mechanisms of action (activation markers, signaling pathways…)
Personalized
approach
- Targeted discussion based on your request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- Custom cellular models: over 100 human cancer cell lines available, to be cultured with target primary immune cells
Tell us about your project !

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tumor antigen-specific T cell killing assays I in vitro CRO services
In addition to the fast T cell response to tumor cells, specific recognition of tumor-specific antigens by cytotoxic T cells – where tumor antigen presentation by APCs such as dendritic cells (DCs) is a crucial step for the induction of effector T cell-mediated killing phase – is the foundation of cancer immunotherapy. The therapeutic potential of increasing DC functionality and tumor antigen sensing in priming antitumor immunity may represent an effective strategy to improve current therapies and develop innovative targeted ones.