The underlying question: Does your compound enhance germinal center reaction by modulating specialized B – T cells interactions and responses?
- Format: 96-well plates
- Model: co-culture of autologous follicular helper T cells (Tfh) and memory B (Bmem) cells from tonsils removed upon surgical intervention
- Readout: IgG released levels as a surrogate of B cell differentiation into plasma cells
- Standards: Staphylococcal enterotoxin B (SEB) – activator, anti-PD1 (Nivolumab), anti-PDL1 (Atezolizumab), anti-CTLA4 (Ipilimumab), anti-CD40 antibody (Bleselumab)
- On request: flow cytometry immunophenotyping (activation markers, immune checkpoint expression…), cytokine release, MoA and signaling pathways deciphering, proteomics, transcriptomics
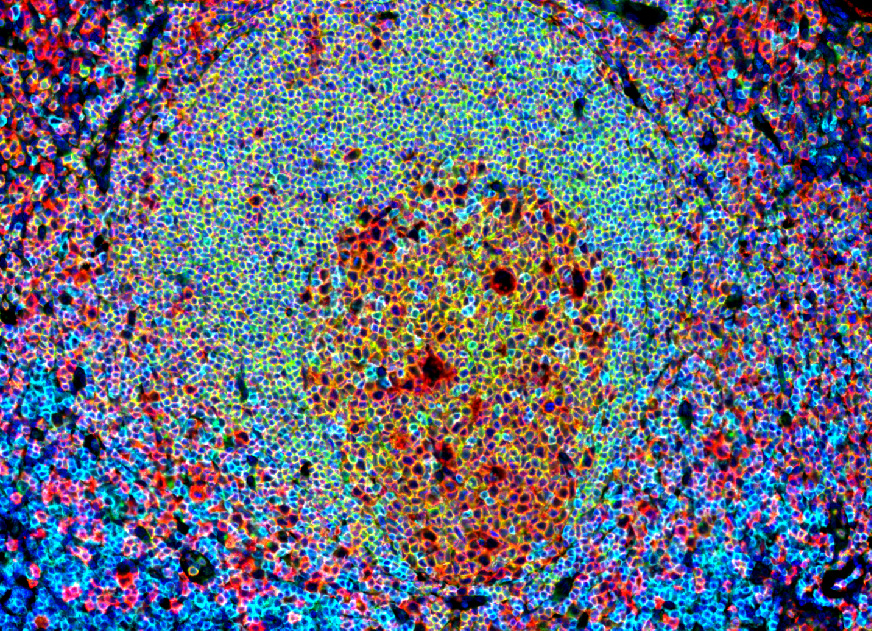
Immunohistofluorescence field of a human tonsil section illustrating the germinal center in a lymphoid structure. This image, obtained on our digital pathology platform, highlights the presence and organization of interactive Tfh (CD4+/CXCR5+) and B cells (CD20+). These features make tonsils our tissue of choice for isolating Tfh (CD4+/CXCR5+) and memory B (CD19+/CD27+) cells for our in vitro assay.
Assay principle
- Co-culture of human autologous follicular helper T cells and memory B cells, treated with test compound alone, or in combination with immune checkpoint inhibitors or chemotherapies.
- Activation stimulus: with Staphylococcal enterotoxin B (SEB)
- Measurement of IgG levels in supernatant after 7 days using HTRF, to assess memory B cell activation and differentiation into plasma cells
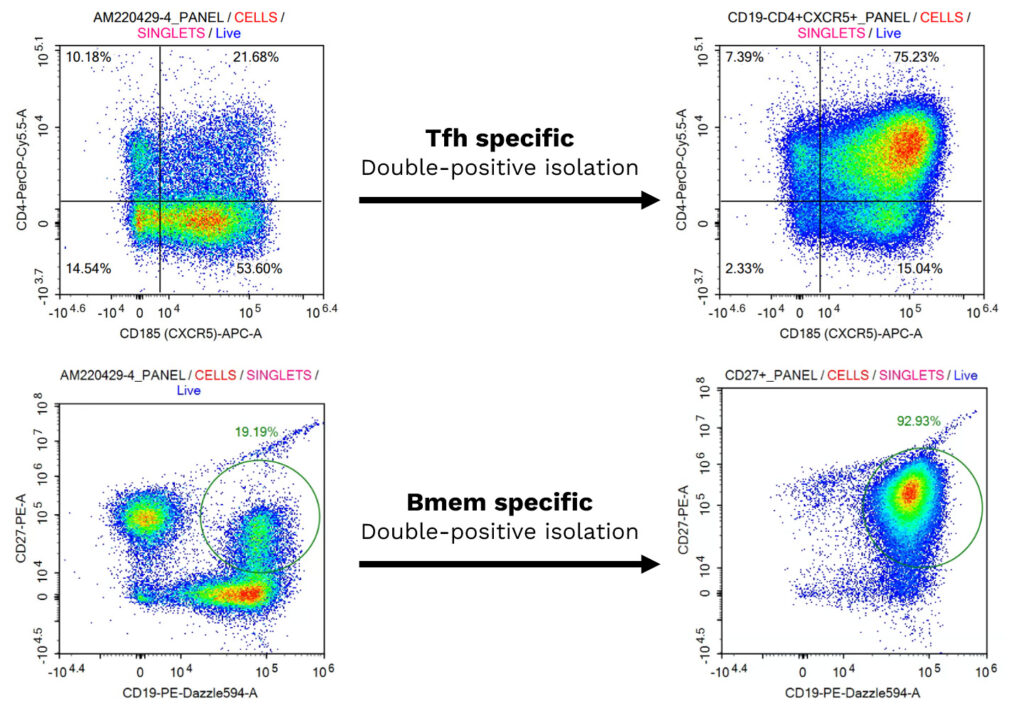
Isolation and Characterization of Primary Human Follicular Helper T cells and Memory B Cells from a Tonsil Donor
(A) Freshly isolated follicular helper (Tfh) T and memory B (Bmem) cells were characterized by multiplexed flow cytometry. Tfh cells were identified as CD4+/CXCR5+, and Bmem cells as CD19+/CD27+, both before and after isolation, confirming their enrichment. (B) The co-cultured Tfh and Bmem cells were visualized using phase contrast imaging to monitor cell interactions overtime.
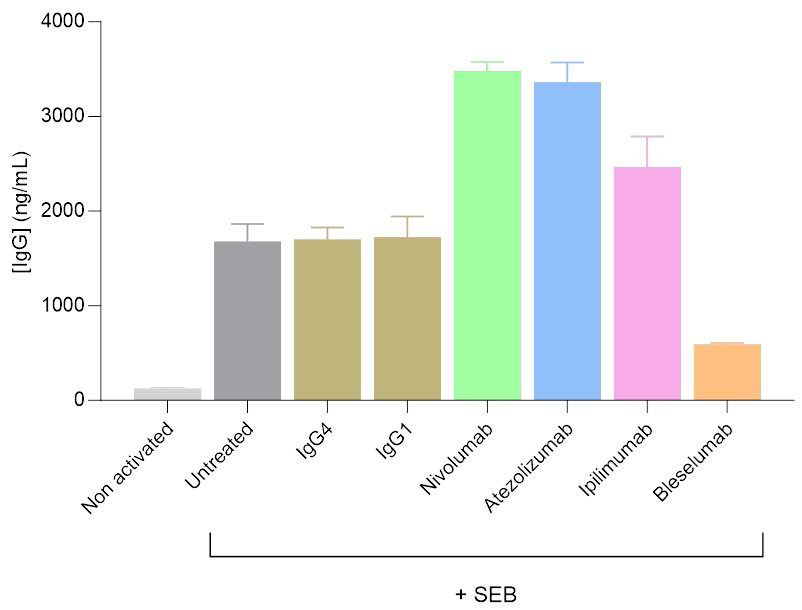
Activation and modulation of IgG secretion by Tfh/Bmem Cell co-culture treated with immunomodulatory antibodies
Isolated T follicular helper (Tfh) and memory B (Bmem) cells were co-cultured, and IgG levels in the supernatants were measured after 7 days using a HTRF-based assay. SEB served as the primary activator of the Tfh/Bmem synapse. SEB was used alone or in combination with antibodies targeting the modulation of this interaction. As reflected by changes in IgG secretion levels, antibodies including anti-PD1 (Nivolumab), anti-PDL1 (Atezolizumab), and anti-CTLA4 (Ipilimumab) were found to enhance Tfh-mediated activation of Bmem cells, whereas the anti-CD40 antibody (Bleselumab) reduced Tfh-mediated Bmem cell activation.
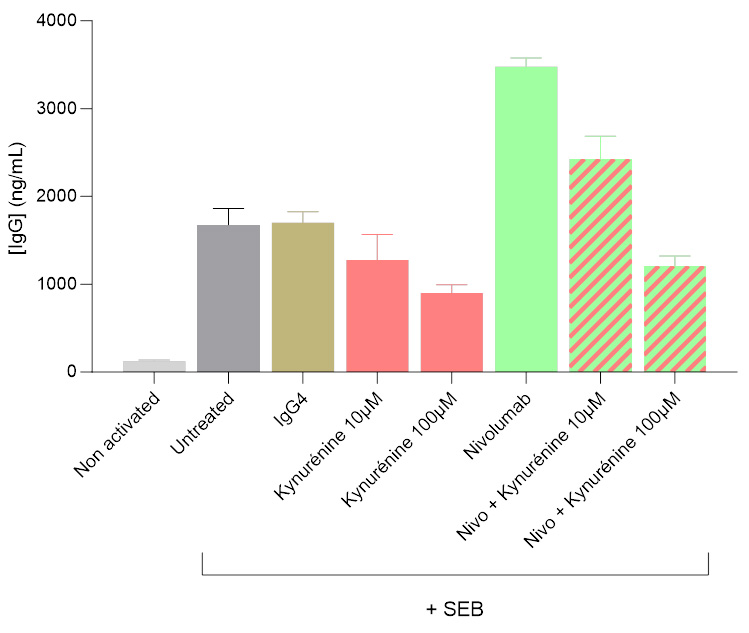
Kynurenine-mediated inhibition of IgG secretion in a Tfh/Bmem co-culture system
Isolated Tfh and Bmem cells were co-cultured, and IgG levels in the supernatants were quantified 7 days post-initiation using a HTRF-based assay. Kynurenine was shown to significantly modulate the activation of the Tfh/Bmem synapse, initially triggered by SEB. Both in the presence and absence of the anti-PD1 antibody Nivolumab, kynurenine led to a dose-dependent decrease of IgG secretion, illustrating its inhibitory effect.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 100+ in vitro campaigns conducted over the past 10 years
- A specific expertise in TLS based on digital pathology and spatial transcriptomics
- 30+ peer-reviewed publications in key immuno-oncology journals
Personalized
approach
- Targeted discussion based on your request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- A flexible technology platform for in-depth mechanism-of-action studies
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
TLS Germinal Center Reaction Assay I In vitro Tertiary Lymphoid Structure Modulation Assay I Cancer Immunotherapy CRO Services
Increasing evidence suggests that the presence of Tertiary Lymphoid Structures (TLS) within tumors is specifically associated with favorable outcomes upon treatment with immune checkpoint blockers (ICB) unlike chemotherapy (4). These results support using TLS as a biomarker to select cancer patients who might benefit from immunotherapy and underscore for the crucial role of B cells in response to ICB (5).
TLS are ectopic lymphoid structures, that develop at sites of chronic inflammation – including cancers – in non-lymphoid tissues. They are characterized by a high density of B cells and plasma cells producing specific antibodies targeting tumor-associated antigens. Within TLS, B lymphocytes stimulated by T follicular helper cells (Tfh) differentiate into Plasma Cells (PC) producing IgG, IgA, or IgM. The presence of IgG+ PCs within tumors confers a positive prognostic value across a wide spectrum of indications, prompting interest in their specific induction as a novel immunotherapy form or as a strategy to enhance effectiveness of current immunotherapies.Given the significance of TLS in response to ICB, we decided to develop a TLS activation assay. This assay involves the specific isolation and co-culture of autologous Tfh and memory B (Bmem) cells and quantifies IgG levels as a standard surrogate for synapse activation and associated PC differentiation. This in vitro functional system replicates key features of TLS and is specifically designed to assess the activity of new immunotherapeutics that stimulate this synapse.