Nature Cancer, 2021
Mature TLS predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression

Nature Medicine, 2022
Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort

Clinical Cancer Research, 2023
Upregulation of IDO1 in tumor cells and Tertiary Lymphoid Structures is a hallmark of inflamed Non–Small Cell Lung Cancer
Cell Reports Medicine, 2025
Spatial transcriptomics reveal the determinants of primary resistance to immunotherapy in NSCLC with mature tertiary lymphoid structures
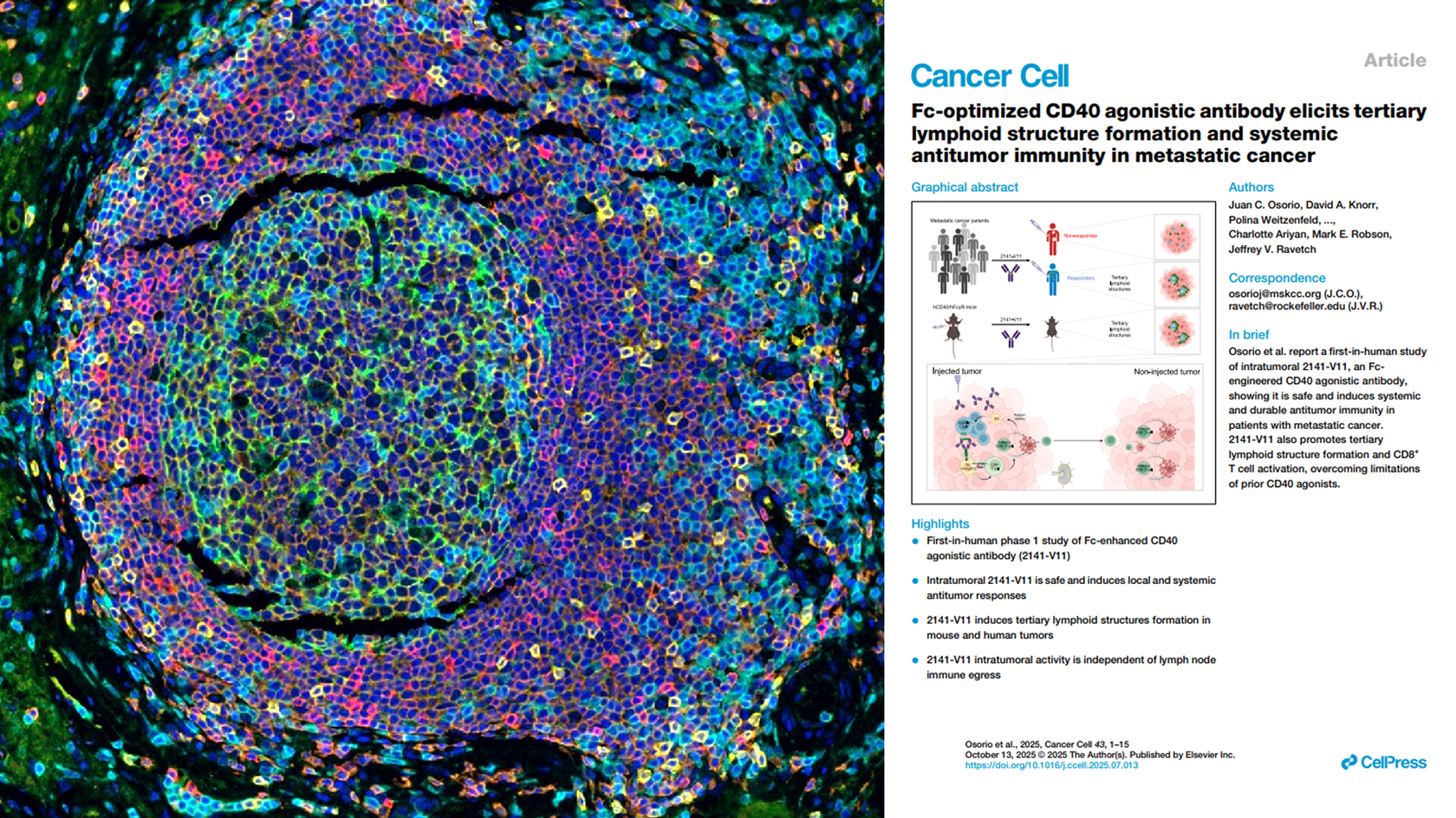
Cancer cell (2025)
Multiplex IHF staining, enabling direct visualization of TLS formation in tumor tissues (MSKCC collaboration)

Processing of tumor specimens
- Eligible samples: formalin-fixed paraffin-embedded (FFPE) tumor tissues specimens
- Source: provided by sponsor or acquired by our team
- In-house capacities: fixation, paraffin embedding, slicing & slide preparation, H&E coloration & annotation by trained pathologists

Multiplexed TLS imaging
- In-house validated panels (IHF & IHC-based) to evaluate TLS presence & maturity
- Automated staining with Ventana Discovery XTTM systems
- Large-capacity multispectral imaging with PhenoImagerTM systems
Expert pathologists for TLS scoring
- Expert pathologists to determine TLS presence & maturity status using serial IHF/IHC & HES images
- Extensive experience in TLS scoring with > 10,000 cases already evaluated across different tumor types
- Delivery of a TLS report per case (request sample report)
Assessment of the presence of TLS and their maturation stage in tumors
Illustration of a mature TLS detected in a primary pancreatic adenocarcinoma.
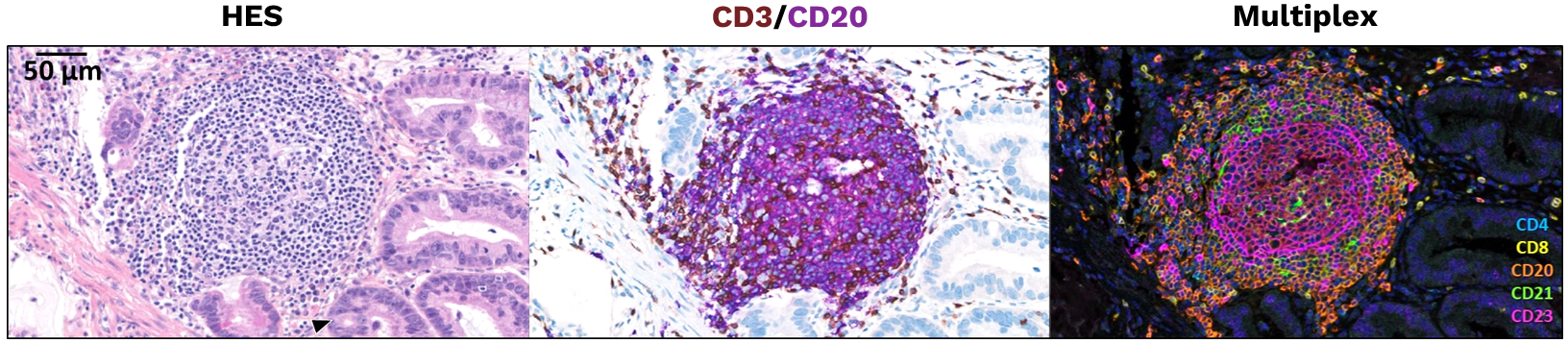
Illustration of an immature TLS detected in a primary lung adenocarcinoma.
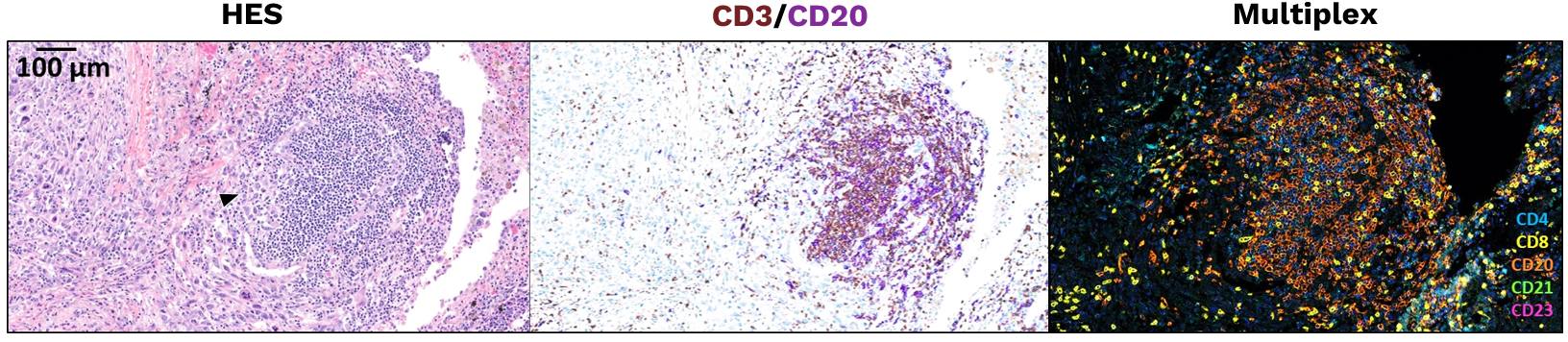
This panel shows representative examples of immature and mature TLS observed in tumor samples. The pictures from the left to right column correspond to 1) Hematoxylin Eosin Saffron (HES) staining, 2) Double immunohistochemistry staining of CD3/CD20 (CD3 in brown, CD20 in purple), 3) Multiplex immunofluorescence assay of CD4 (blue), CD8 (yellow), CD20 (orange), CD21 (green) and CD23 (pink). The scale bars on the HES images indicate 50µm and 100µm for the upper and lower panels, respectively. Black cropped arrows highlight the tumor cells in the samples.
Mature TLSs are predictive of outcome in patients with cancer treated with immune checkpoint inhibitors
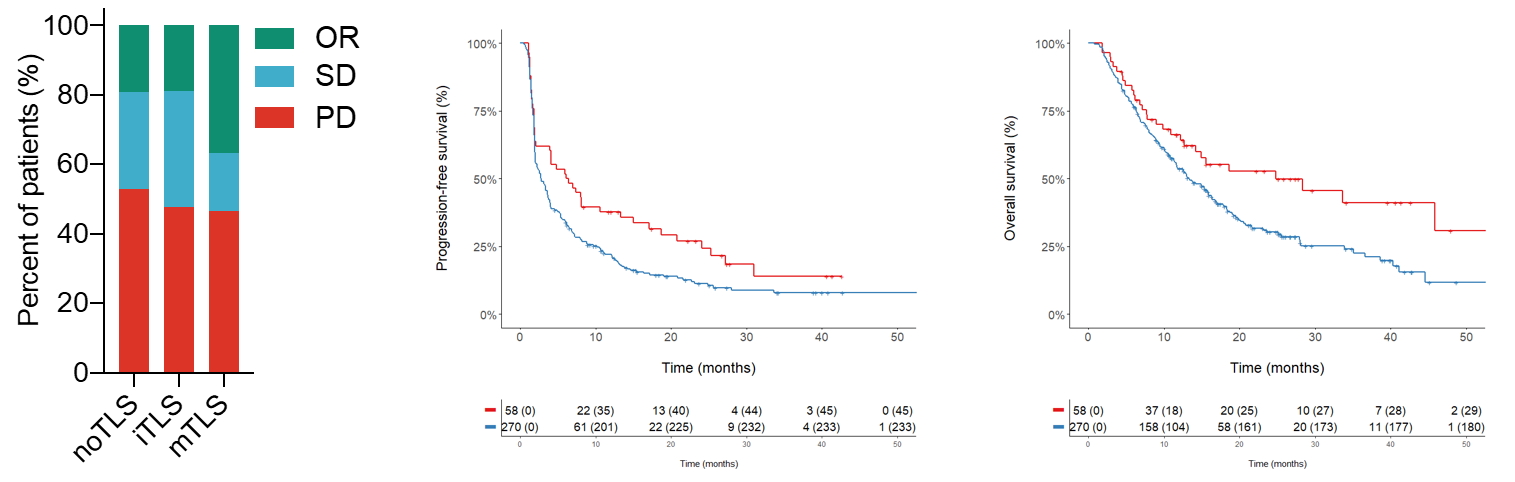
The presence and maturity of TLSs were assessed in TLS-positive tumors using a multiplex immunofluorescence assay combining CD4, CD8, CD20, CD21 and CD23 markers. The results are representative of 540 tumors analyzed. Left: Response rate, as defined per RECIST 1.1 criteria, according to TLS status: absence (no TLSs; n = 223 patients), iTLSs (n = 21 patients) or mTLSs (n = 84 patients). OR, objective response; PD, progressive disease; SD, stable disease. Statistical significance was determined by chi-squared test. Middle and right: Kaplan–Meier curves of progression-free survival and overall survival in the discovery cohort of 328 patients with cancer who were treated with anti-PD-1 or anti-PD-L1 antagonists according to mTLS status (red curve: mTLS-enriched tumors; blue curve: mTLS-negative tumors). Numbers below each x-axis indicate the number of patients at risk and those in parentheses are the number of events. Statistical significance was determined by log-rank test).
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (translational study director)
Tell us about your project !
TLS Detection & Scoring for Cancer Immunotherapy
In the past few years, conjointly with teams from Institut Bergonié (Pr Antoine ITALIANO, Dr Lucile VANHERSECKE), Explicyte Immuno-Oncology released papers in Nature Cancer, Laboratory Investigation, and Nature Medicine, highlighting the importance of TLS analysis in the context of cancer immunotherapies. We thus developed a multiplexed panel to detect TLS presence and maturity status to ultimately select patients who might benefit the most from cancer immunotherapies. Based on in-house capacities in digital pathology (from sample preparation to automated acquisition and scoring), we launched a dedicated service for the scoring of TLS in cancer patient biopsies.