-
- Model features: CD8 cell-mediated control of tumor growth, lymphoid & myeloid immune tumor infiltration
- Tumor cell line: MCA205 tumor cells (parental or PD1/PDL1 resistant)
- Tumor implantation: subcutaneous
- Standards: immune checkpoint inhibitors (anti-PD1, PDL1, CTLA4), doxorubicin
- Readouts: body weight, tumor size, survival
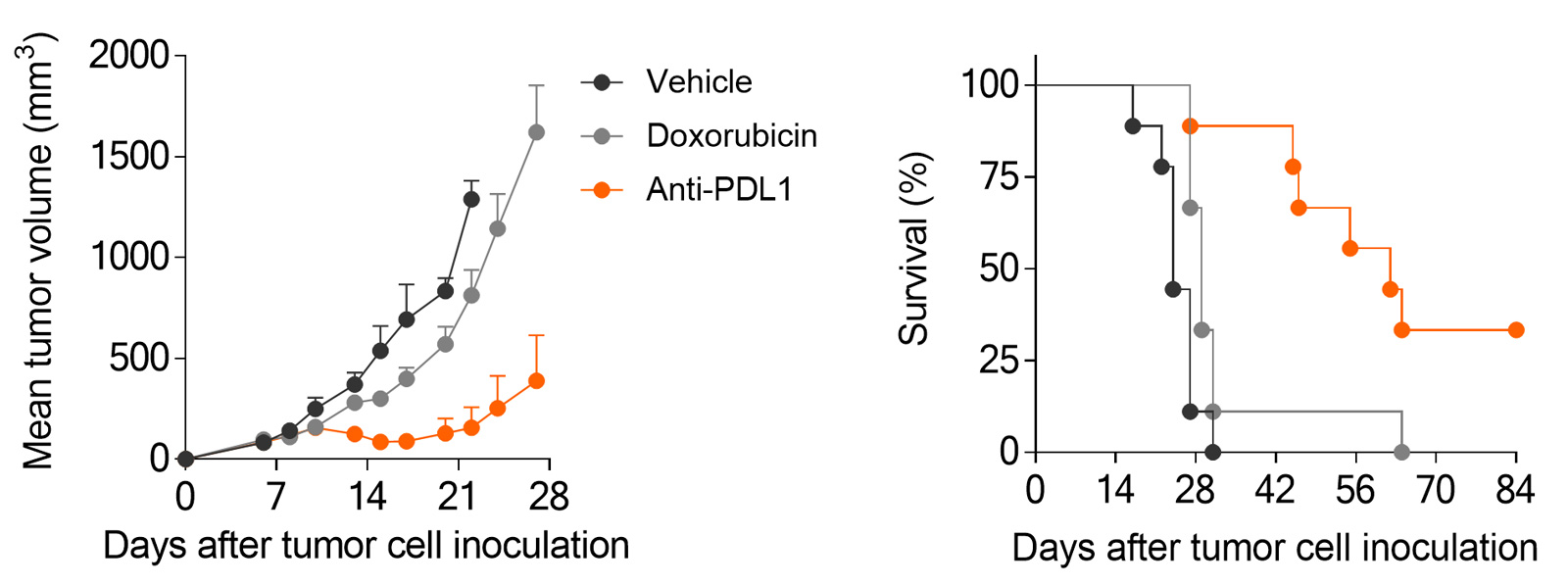
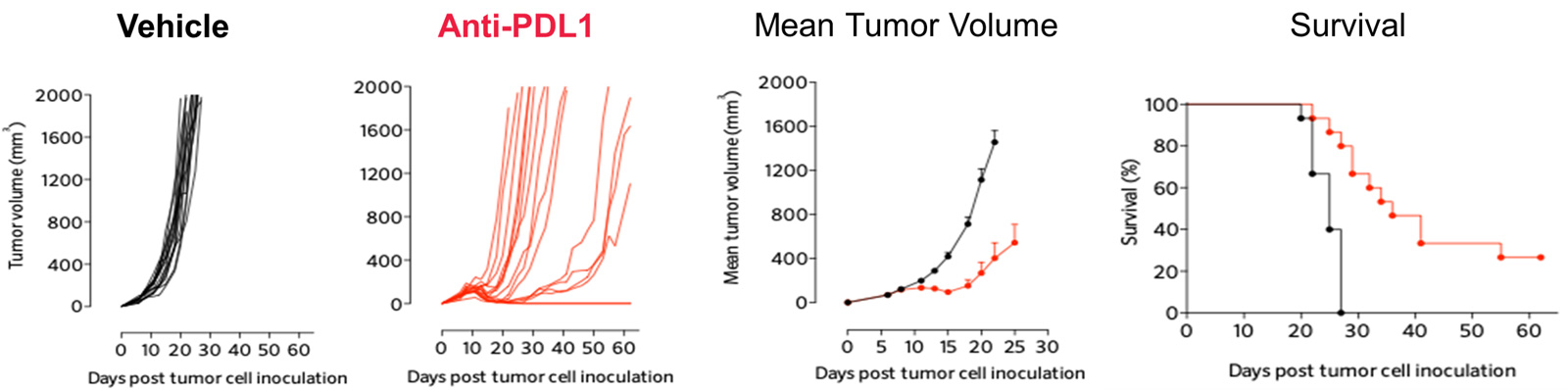
Subcutaneously-implanted MCA205 sarcoma tumor-bearing mouse model is strongly responsive to PD1/PDL1 blockade and only partially to doxorubicin.
In vivo efficacy & mechanism of action studies for novel immunotherapies
Straightforward in vivo efficacy studies
- N=10: Standard groups of 10 mice including groups exposed to test compound alone and in combination with reference therapy.
- Weekly reports: monitoring tumor growth, body weight, and survival
Flexible sampling options
- Monitoring response over time: satellite mice, serial bleeding, intra-tumoral biopsies
- On-demand sample collection: blood, serum, plasma, tumor, organ samples
A flexible platform to quantify tumor-microenvironment & peripheral markers
- Multiplex immunophenotyping by flow cytometry & digital pathology
- Spatial transcriptomics & proteomics
MCA205 sarcoma model is responsive to PD1/PDL1 blockade, while still providing dynamic range for optimization
Anti-tumor effects of Immune checkpoint inhibitors as single agent or in combination.
Mice were subcutaneously inoculated with MCA205 tumor cells and treated with either vehicle, anti-PD1, anti-PDL1, anti-CTLA4 or with a combination of i) anti-PD1 plus anti-PDL1, ii) anti-PDL1 plus anti-CTLA4 or iii) with anti-PD1 plus anti-CTLA4. Tumor growth and survival were monitored overtime.
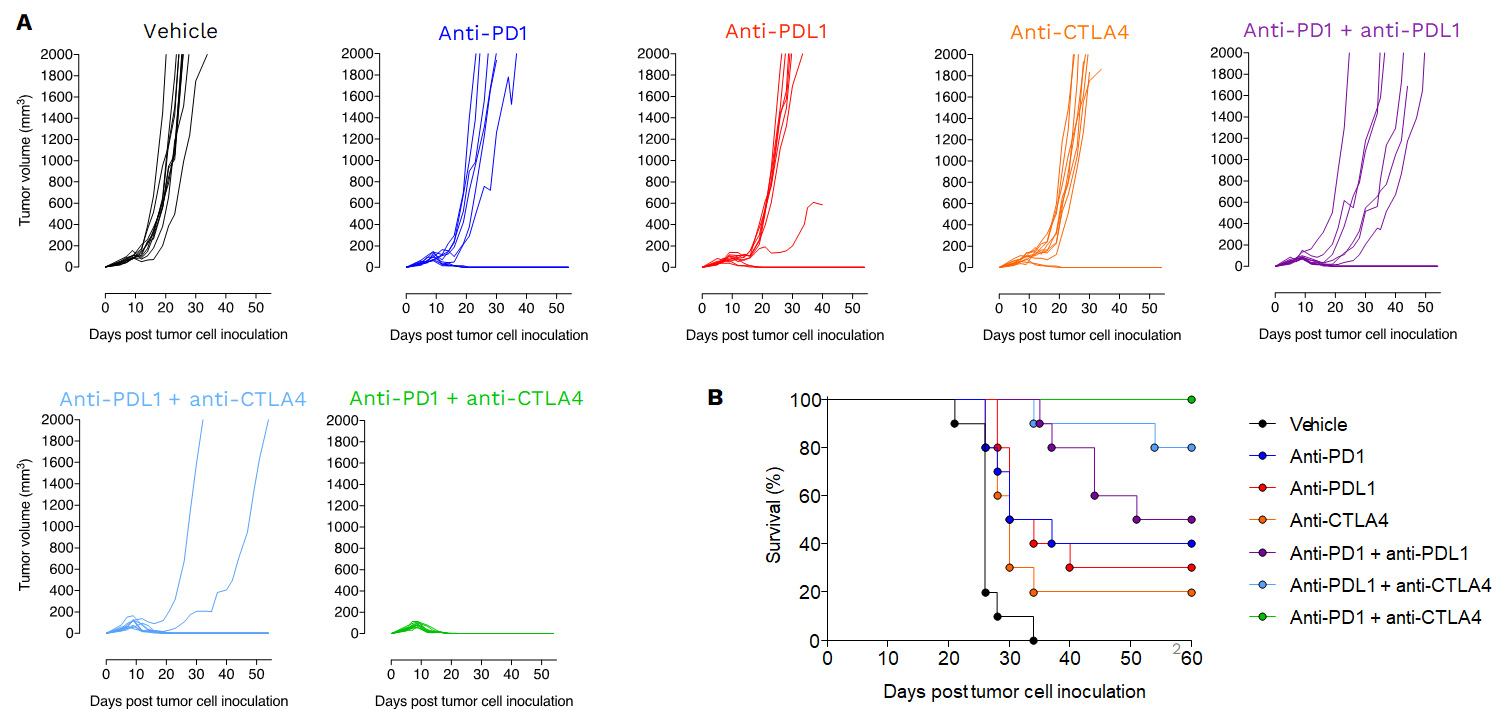
PD1/PDL1 blockade enhances antitumor immune response reprogramming in the MCA205 sarcoma model
PDL1 inhibition triggers significant anti-tumor response in the MCA205 sarcoma mode
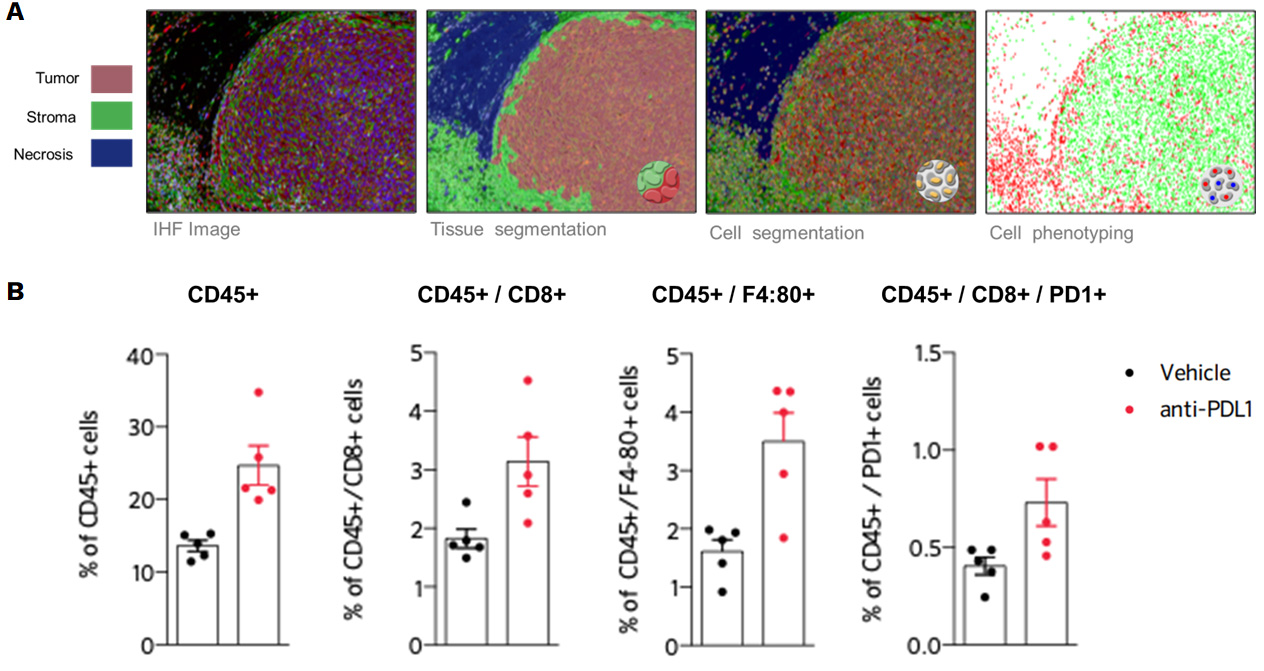
Blocking PDL1 promotes recruitment of lymphocytes and macrophages within the tumor (B), highlighted by immunohistofluorescence-based image analysis of the immune cell infiltrate, depicted in (A).
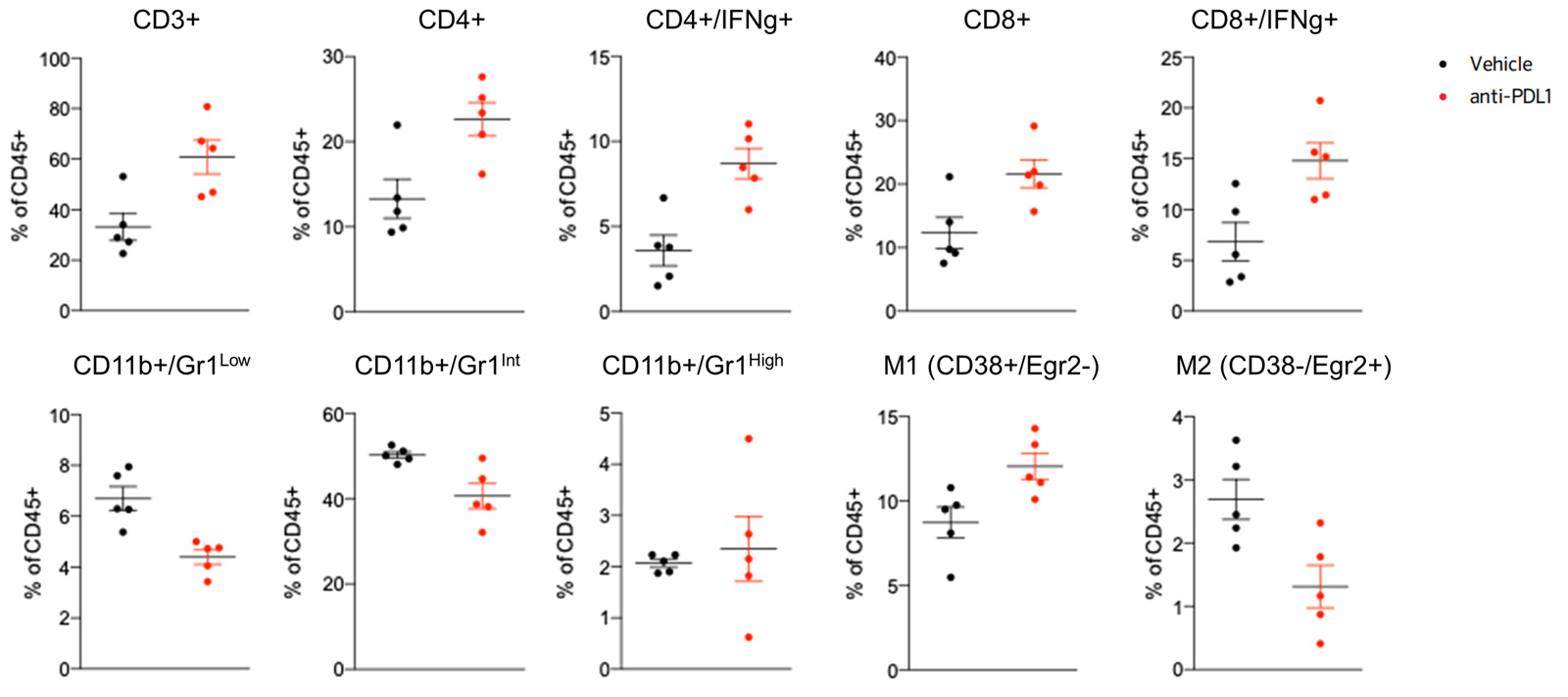
Blocking PDL1 favors recruitment of lymphocytes and M1 macrophages while decreasing M2 macrophages and MDSCs, within the tumor, evidenced by flow cytometry-based analysis of the immune cell infiltrate.
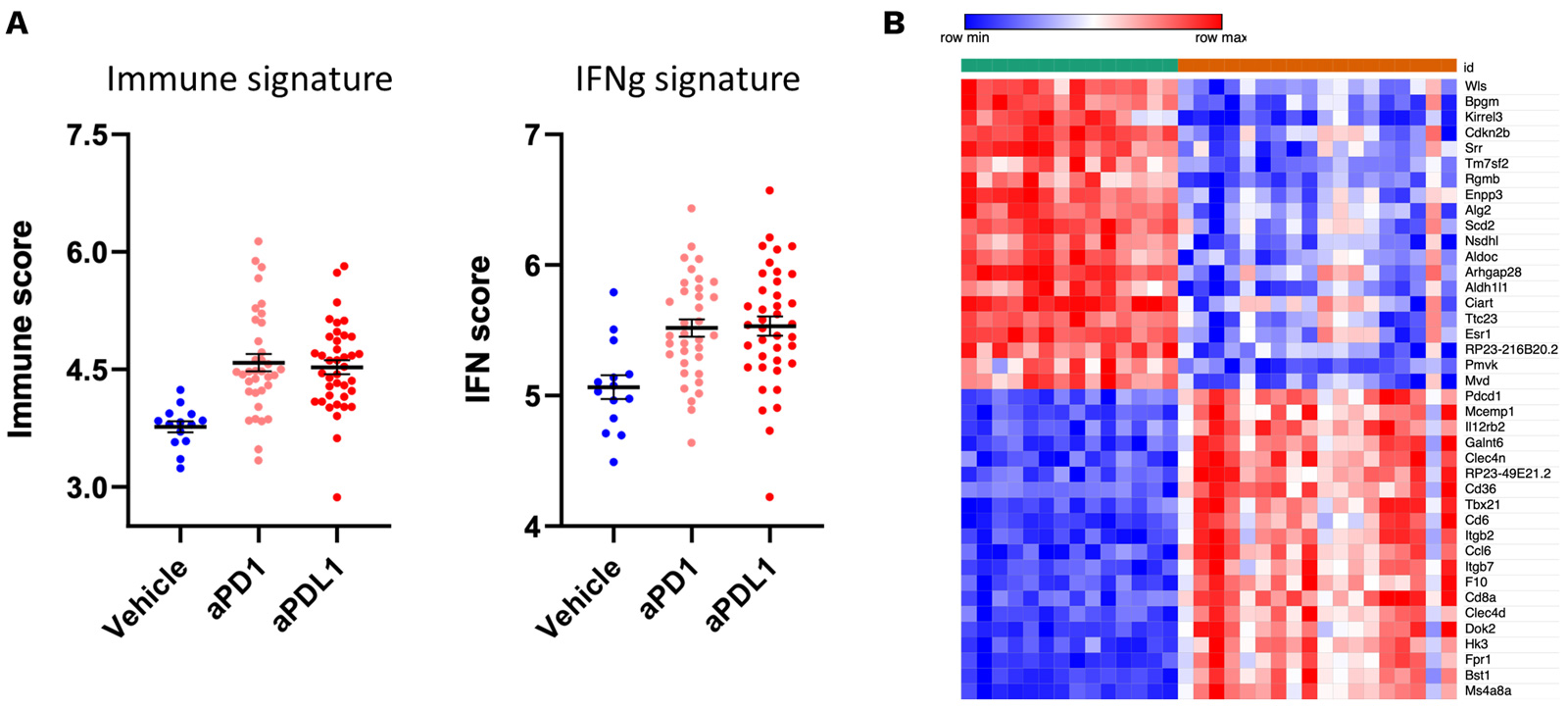
Antitumor effects of PD1/PDL1 inhibitors are underpinned by an IFNg and immune signature.
RNA sequencing of MCA205 tumor biopsies collected under vehicle and PD1/PDL1 blockade highlights a specific gene expression signature profile associated with anti-PD1/PDL1 treatments characterized by an IFNg and immune signature (A). Top 20 upregulated and downregulated genes is displayed in (B).
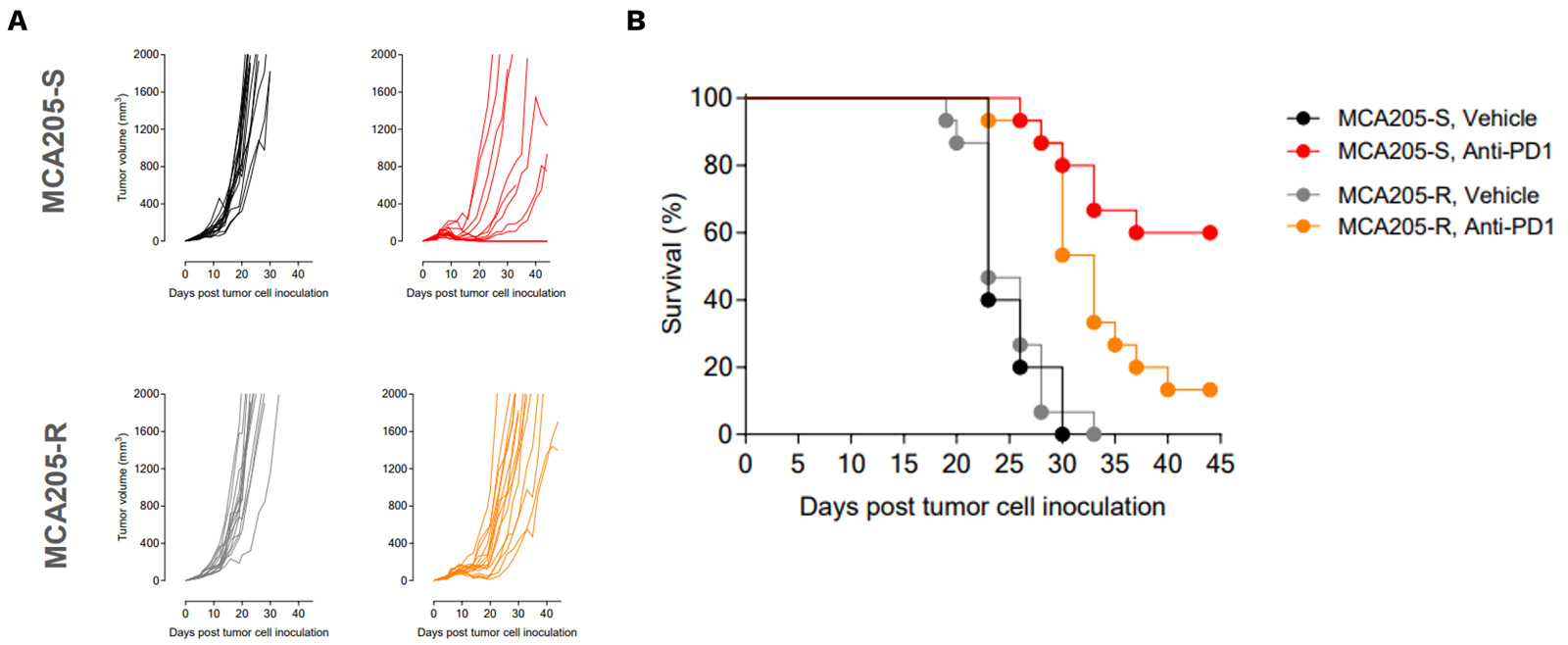
PD1/PDL1 resistant MCA205 model exhibits only partial and very moderate sensitivity to PD1 blockade
PD1/PDL1 resistant MCA205 model (MCA205-R) is strongly less sensitive to PD1 blockade than MCA205 wild-type (MCA205-S) tumors.
Mice were subcutaneously inoculated with MCA205-S (wild-type) or MCA205-R (PD1/PDL1 resistant, developed in-house) tumor cells and treated with either vehicle or anti-PD1. Tumor growth and survival were monitored overtime.
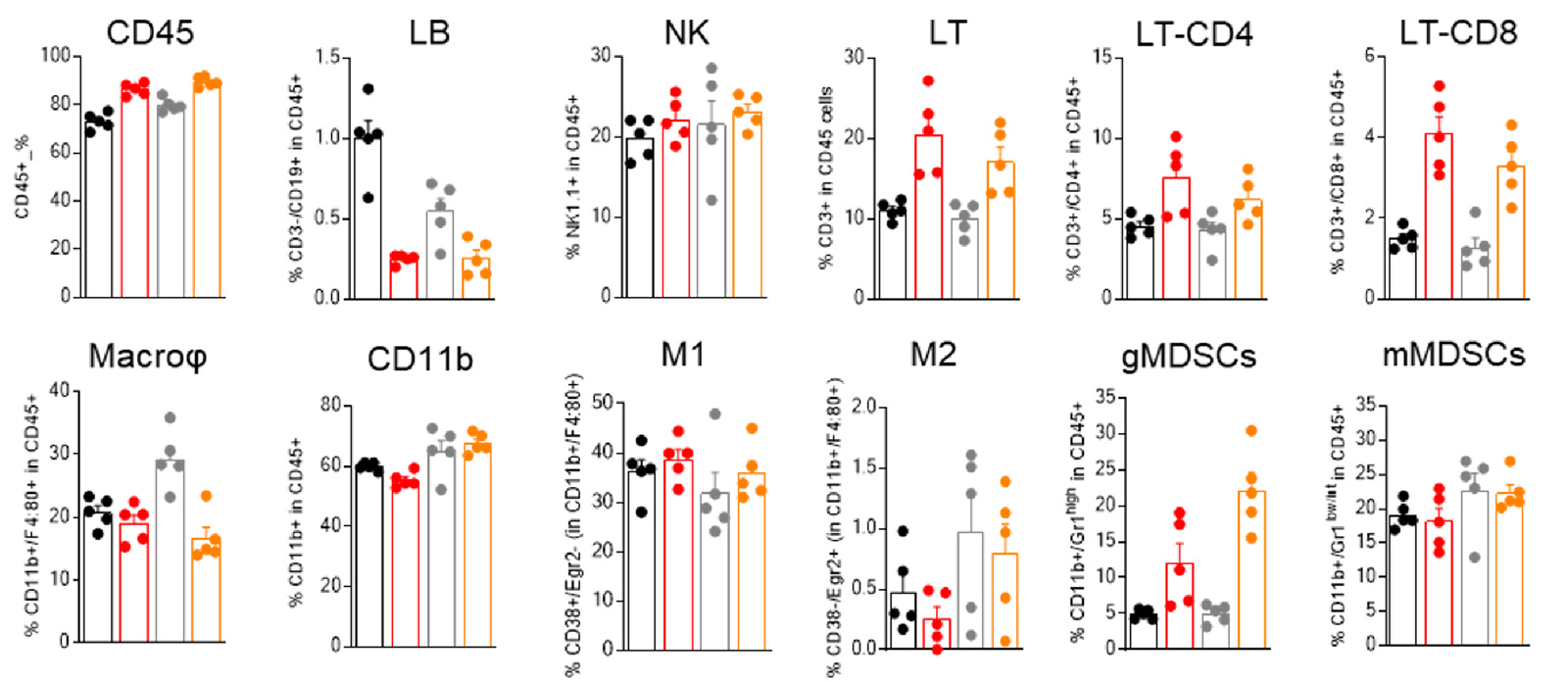
MCA205-R and MCA205-S tumors are featured of different baseline immune infiltrate, differentially modulated upon PD1 blockade
Flow-cytometry-based analysis of MCA205-S (wild-type) and PD1/PDL1 resistant (MCA205-R) tumor immune cell infiltrate, upon vehicle or anti-PD1 treatment.
Analysis of the tumor immune infiltrates reveals, particularly, a greater abundance of macrophages in MCA205-R than in MCA205-S tumors, and underlines a differential modulation of immune cell subsets within MCA205-S and MCA205-R tumors upon PD1 treatment.
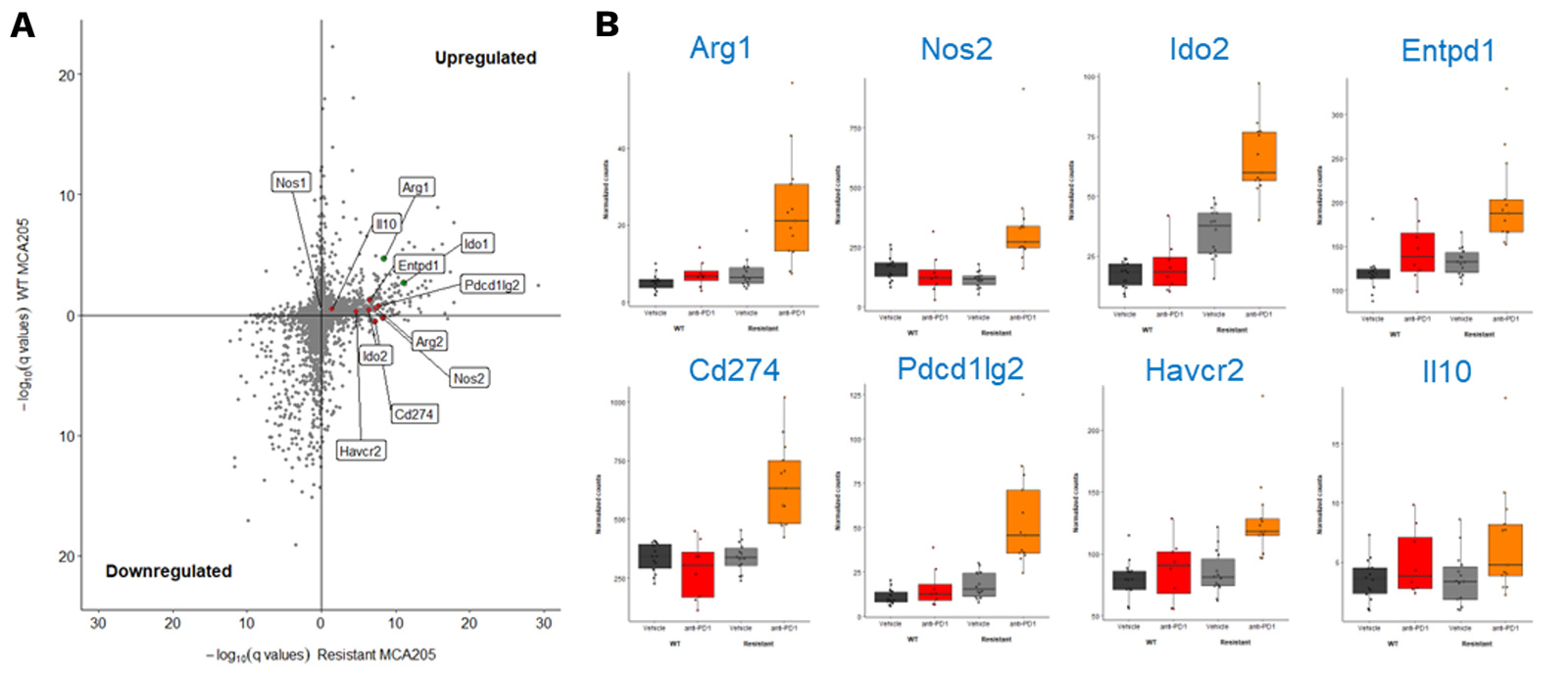
MCA205-R tumor model exhibit a key gene expression profile differentially modulated upon PD1 blockade
RNA sequencing of MCA205-S and PD1/PDL1 MCA205-R tumor biopsies collected under vehicle and PD1 blockade highlights key genes significantly upregulated upon PD1 inhibition in MCA205-R tumors.
(A) Volcano plot depicting significativity of changes in gene expression between vehicle and anti-PD1 treated MCA205-S (Y axis) and MCA205-R (X axis) tumor-bearing mice. Red dots show some myeloid-related genes significantly upregulated only in anti-PD1 treated MCA205-R tumor-bearing mice while green dots indicate gene differentially upregulated in both MCA205-S and MCA205-R mice. (B) Histograms illustrating the differential expression of some of these genes between vehicle and anti-PD1 treatment, in mice carrying MCA205-S or MCA205-R cells.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 150+ in vivo campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- Bespoke study designs based on client objectives and literature
Personalized
approach
- A dedicated study director (PhD level) from experimental plan to final report
- Weekly reports to provide regular updates & adapt experimental strategy
- Comprehensive analytical platform to decipher anti-tumor response
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
MCA-205 Syngeneic Mouse Model of Sarcoma I In Vivo CRO Services I Cancer Immunotherapy CRO
Current immunotherapies, especially immune checkpoint inhibitors such as PD1 / PDL1 axis blockers (Nivolumab, Pembrolizumab, Atezolizumab, etc.), have dramatically changed the clinical outcome of cancer patients and constitute now an important part of the therapeutic armamentarium. However, despite their success, the benefit of cancer immunotherapies remains limited to a fraction of patients and varies according to the tumor histotype as well as with the intrinsic features of the tumor (eg. Tumor Mutational Burden, PDL1 expression, etc.) or its microenvironment (immune cell infiltration, fibroblast abundance, etc.). As to increase the current rate of response, several approaches are under evaluation and include combination therapies targeting other immune checkpoints (CTLA4, TIGIT, LAG3, etc.), or immunosuppressive pathways (Adenosine, Kynurenine, Arginine, etc.), etc. Using a syngeneic MCA205 sarcoma tumor model – widely used for the evaluation of innovative cancer immunotherapies – we investigated the anti-tumor activity of several combination therapies. This model is known to be responsive to PD1/PDL1 axis blockade but only partially to anti-CTLA4 antibody. Interestingly, while an additive benefit of anti-PD1 and anti-PDL1 is observed, a clear synergistic effect is achieved upon combination of either anti-PD1 or anti-PDL1 with anti-CTLA4, with a full tumor rejection in all treated animals. These data highlight the suitability and relevance of our robust MCA205 mouse tumor model to assess novel combinatorial strategies – on top of benchmark therapeutics – for their potential synergistic effect, which can ultimately be deciphered through multiple ancillary approaches (tumor immune cell infiltrate and gene expression analysis, digital pathology, etc).










