-
- Model features: aggressive tumor growth, enriched tumor microvascular environment, low immune infiltration, accumulation of circulating MDSCs.
- Tumor cell line: mouse renal adenocarcinoma Renca cell line
- Tumor implantation: subcutaneous
- Standards: Sunitinib (anti-angiogenic), PD1/PDL1 blockers, hypoxia-regulating agents
- Readouts: body weight, tumor size, survival
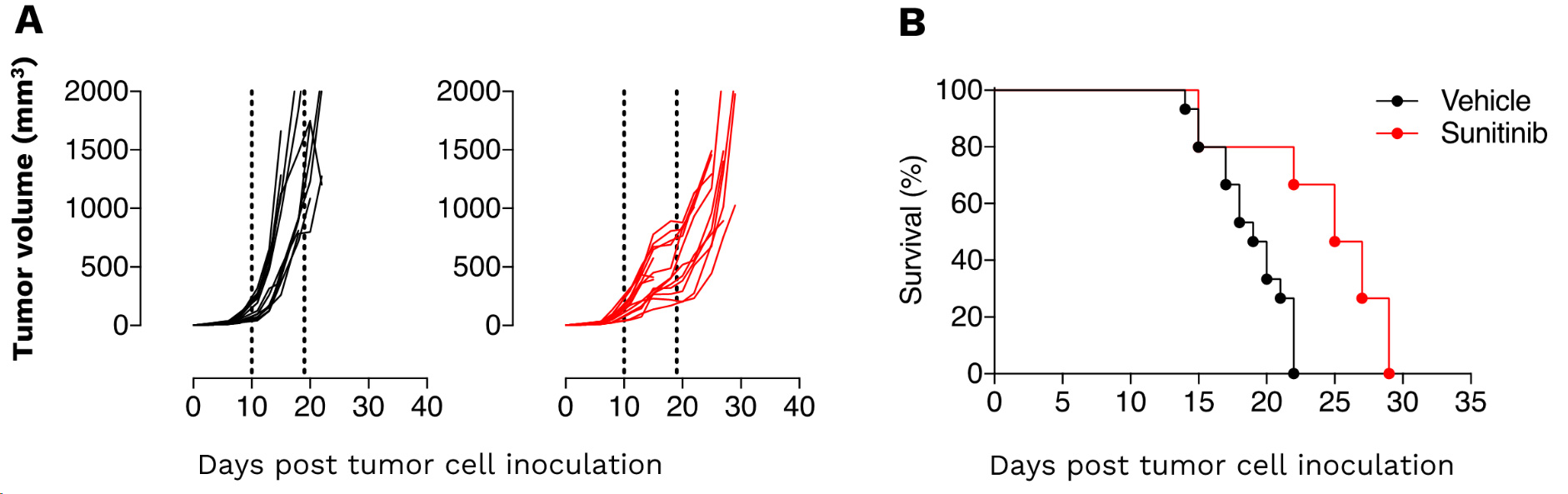
Renal Renca syngeneic tumor model – as a model of renal malignancies known as angiogenesis-dependent – displays a great sensitivity to Sunitinib. (A) Individual tumor volume (mm3) and (B) Kaplan-Meier plot survival of Renca tumor-bearing mice upon vehicle and sunitinib treatment. Renca tumors are significantly responsive to the anti-angiogenic agent. The response amplitude to sunitinib still offers enough range to evaluate strategies including e.g. vascular stabilizing agents, hypoxia regulators, immune checkpoint blockers, and immune modulators targeting immunosuppressive subpopulations.
In vivo efficacy & mechanism of action studies for novel immunotherapies
Straightforward in vivo efficacy studies
- N=10: Standard groups of 10 mice including groups exposed to test compound alone and in combination with reference therapy.
- Weekly reports: monitoring tumor growth, body weight, and survival
Flexible sampling options
- Monitoring response over time: satellite mice, serial bleeding, intra-tumoral biopsies
- On-demand sample collection: blood, serum, plasma, tumor, organ samples
A flexible platform to quantify tumor-microenvironment & peripheral markers
- Multiplex immunophenotyping by flow cytometry & digital pathology
- Spatial transcriptomics & proteomics
Sunitinib enhances vascular response in the tumor microenvironment
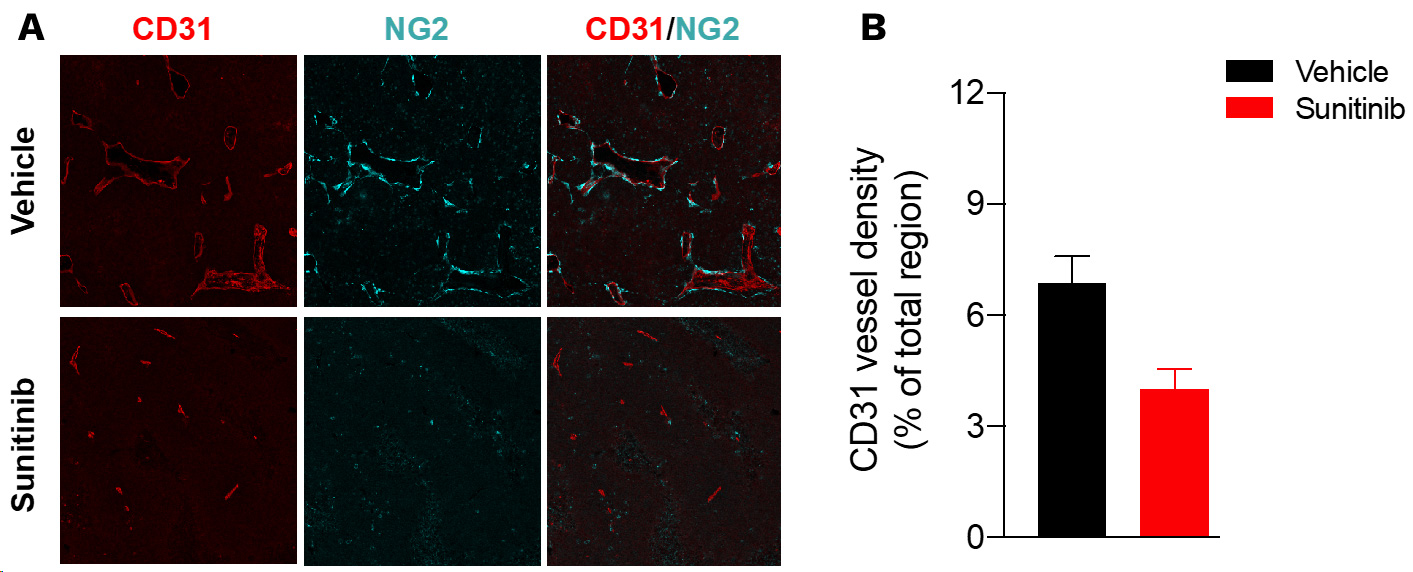
Sunitinib induces tumor vessel density reduction in Renca tumors.
Renca tumor-bearing mice were treated with vehicle or sunitinib. At the end of treatment, tumors were collected and processed for CD31 (red, blood vessels) and NG2 (cyan, pericytes) immunohistofluorescence and image acquisition & analysis. (A) Representative images of CD31 and NG2 immunostaining. (B) CD31 vessel density is analyzed and presented as a percentage per total sectional area.
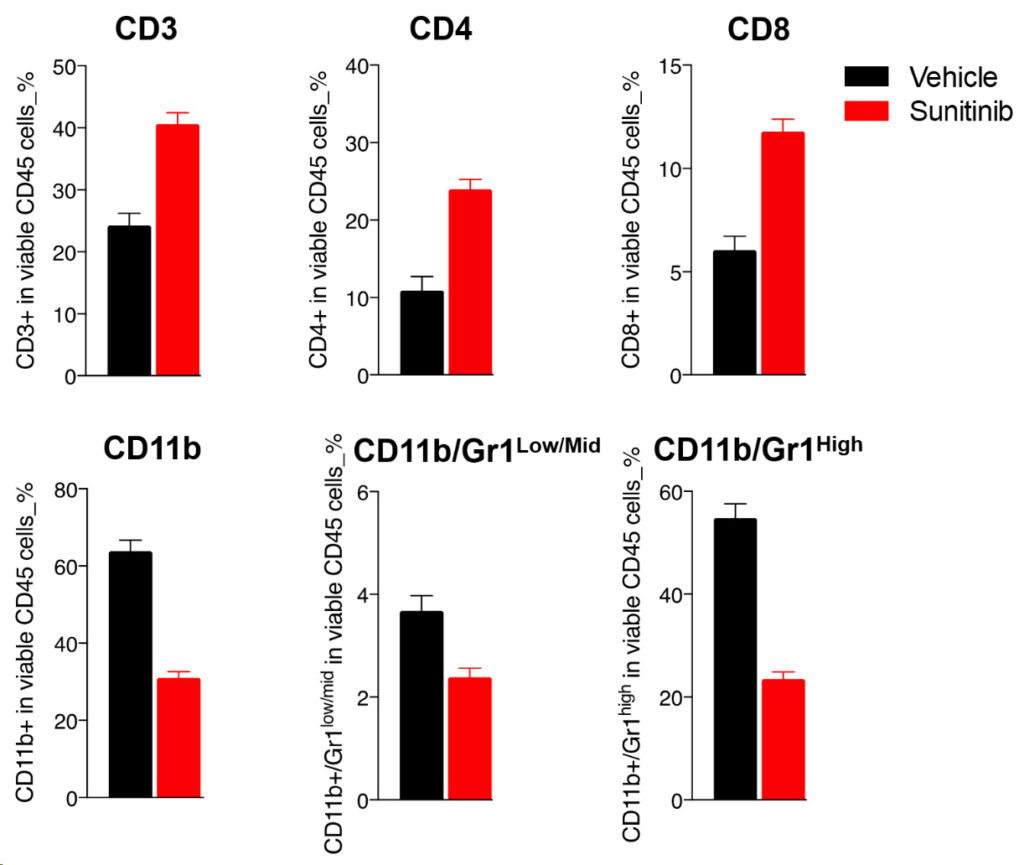
Sunitinib-mediated efficacy is underpinned by a decline in circulating MDSCs and peripheral T cell increase
Multiparametric flow cytometry-based analysis of blood T cells and myeloid cell subsets of Renca tumor-bearing mice upon vehicle, and sunitinib treatment.
Upon sunitinib treatment, blood FACS analysis interestingly shows an important decrease of circulating CD11b myeloid cells and MDSCs, which correlates with a potentially improved peripheral T cell response as underlined by the increase in the CD3, CD4, and CD8 T cell populations.
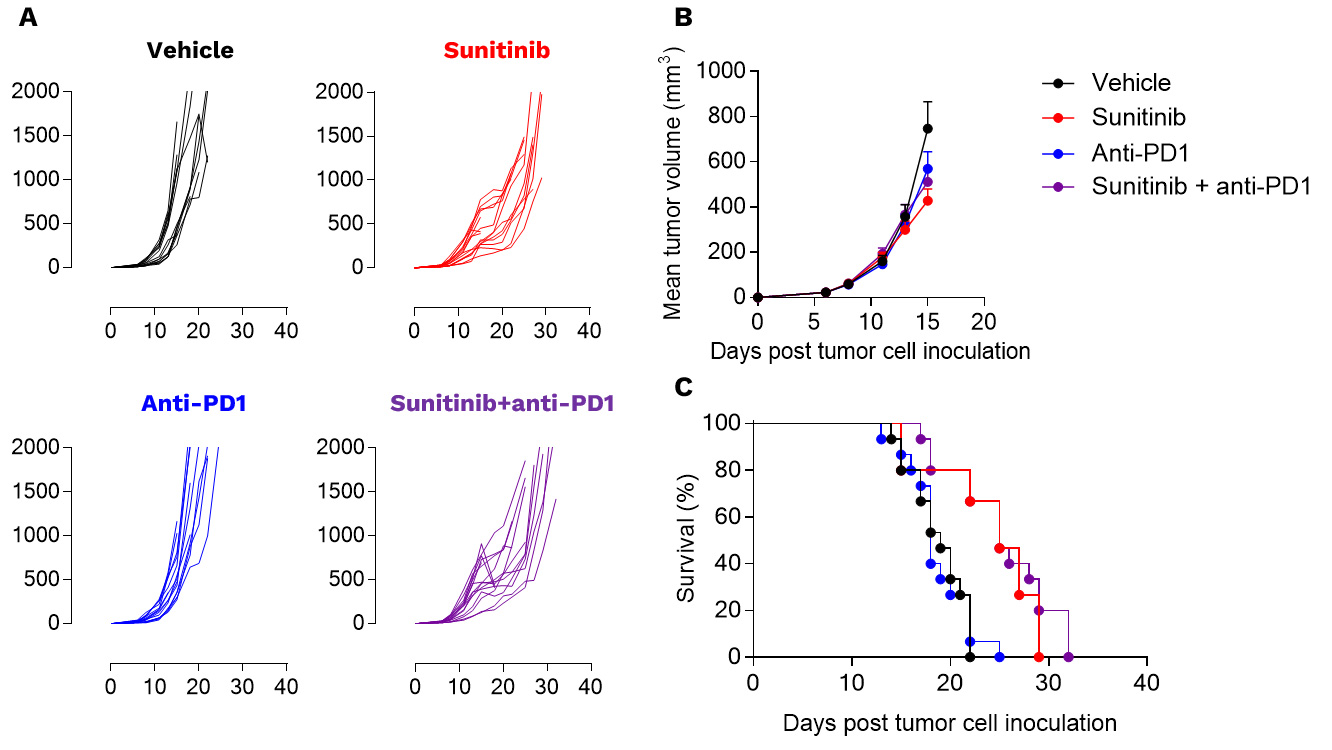
Renca tumors are not responsive to PD1 inhibition
Anti-tumor assessment of sunitinib alone and in combotherapy with PD1 blockade in the preclinical RENCA model. RENCA cells were subcutaneously inoculated and mice were then left untreated or treated with either i) sunitinib or ii) anti-PD1 antibody according to specific treatment schemes. Tumor growth kinetics (A), mean tumor volume (B), and survival (C) curves are depicted. Each graph represents 1 experiment with 15 mice per group.
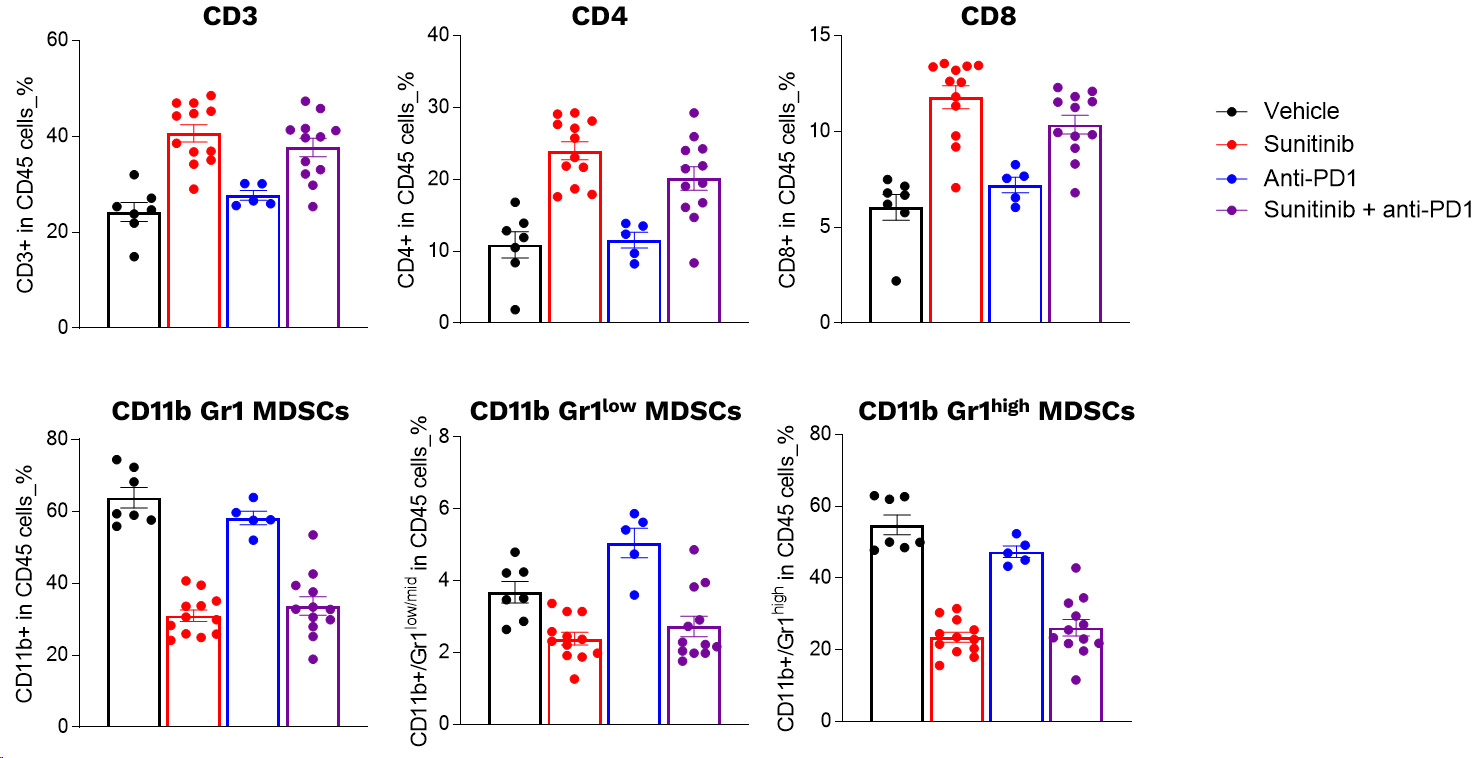
Profiling of circulating leukocytes of Renca tumor model upon sunitinib and PD1 antibody in mono and combotherapy
Profiling of circulating leukocytes upon sunitinib and PD1 blockade. RENCA cells were subcutaneously inoculated and mice were then left untreated or treated with either i) sunitinib or ii) anti-PD1 antibody, according to specific treatment schemes. The day following the last sunitinib dosing, blood samples were collected and processed for multiplexed marker panel immunostaining detection and analysis by flow cytometry. Individual data and means ± SEM are represented.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 150+ in vivo campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- Bespoke study designs based on client objectives and literature
Personalized
approach
- A dedicated study director (PhD level) from experimental plan to final report
- Weekly reports to provide regular updates & adapt experimental strategy
- Comprehensive analytical platform to decipher anti-tumor response
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
Renca Syngeneic Model of Renal Cancer I Kidney Tumor I CRO Services
Renal cell carcinoma (RCC) is the most common kidney cancer subtype with 30% of patients suffering from a metastatic stage at the time of diagnosis. Nevertheless, the development of specifically-targeted therapies e.g. tyrosine kinase inhibitors and anti-angiogeneic agents led in recent years to a significant patient survival improvement. Despite this considerable progress, treatment efficacy still remains to be optimized and new therapeutic strategies are currently investigated consisting in the combination of therapies targeting the tumor microenvironment, and including, among others, immune checkpoint inhibitors. We have fully set up and characterized a syngeneic Renca renal mouse model recapitulating most of the human RCC condition features. Indeed, our model diplays an enriched tumor microvascular environment and relatively low immune infiltration – nevertheless constituted of a strong Myeloid-Derived Suppressor Cells (MDSCs) proportion, which cells serving as pivotal factors of tumor-induced Th2 response, subsequent escape and further promotion of angiogenesis. Furthermore and as in patients, the model shows an accumulation of circulating MDSCs. Interestingly, sunitinib, first-line anti-angiogenic therapy for RCC, leads here to a tumor growth inhibition, an effect seemingly mediated by a tumor vascular normalization and a decline in circulating MDSCs, which correlates with a potentially improved peripheral T cell response. While being an aggressive and non-to-low immunogenic, all the Renca model features make it a powerful immuno-oncology tool whose preclinical development would benefit for the evaluation of new, single or combinatorial, therapeutic strategies.










