The underlying question: Does your compound modulate the dendritic-/T-cell synapse, thereby amplifying anti-tumor immune response, or alleviating immunosuppression?
- Format: 96-well plates
- Model: Allogenic co-culture of primary CD4+ T cells (responder) and monocyte-derived dendritic cells (stimulator)
- Key readout: interferon-gamma (IFNγ) release to assess T cell activation
- Standards: anti-PD1 (Nivolumab, stimulator), anti-PDL1 (Atezolizumab, stimulator), Adenosine receptor ligands (agonists, immunosuppressive, or antagonists, for relief)
- Additional readout: flow cytometry immunophenotyping of dendritic cells (DCs), interleukin production (IL-8, IL-12, IL-10) to assess dendritic cell function before co-culture, proteomics, transcriptomics
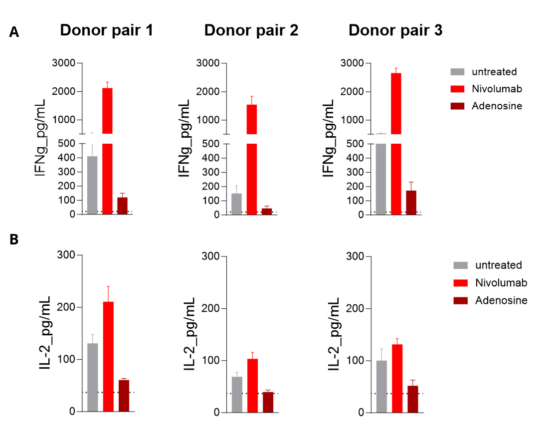
MLR in allogenic human mDC/CD4 T cell co-cultures from independent donor pairs is enhanced upon PD1 inhibition and limited by Adenosine. Isolated peripheral blood monocyte-derived mature DCs are co-cultured with allogenic CD4+ T cells. IFNg (A) and IL2 (B) levels released in the supernatants are then measured by HTRF. While the T-cell stimulating ability of mature DCs is optimized following PD1 blockade with Nivolumab (as the MLR response increases), it’s found to be very limited under Adenosine treatment.
Assay principle
- Co-culture of allogeneic monocyte-derived dendritic cells (donor 1, stimulator) with CD4+ T cells (donor 2, responder)
- Treatment with test compounds during DC differentiation and/or maturation, or at co-culture
- Cytokine release during DC maturation or co-culture (e.g. IL-8, IL-12, IL-10, IFNγ) quantified by HTRF or LEGENDplex™
- Additional readouts: analysis of surface markers and signaling pathways to decipher mechanism(s) of action
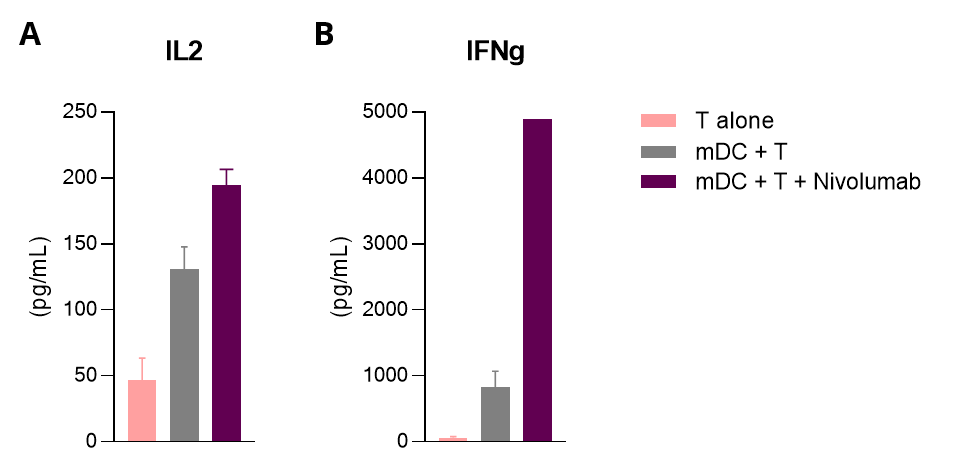
Mixed leukocyte reaction (MLR) in allogenic human mDC/CD4 T cell co-cultures is enhanced upon PD1 inhibition.
Isolated peripheral blood monocyte-derived DCs are co-cultured with allogenic CD4+ T cells. IL2 (A) and IFNg (B) levels released in the supernatants are measured by HTRF. While mature DCs (mDC) display T-cell stimulating ability underlined through the MLR response, this response is further optimized following PD1 blockade with Nivolumab.
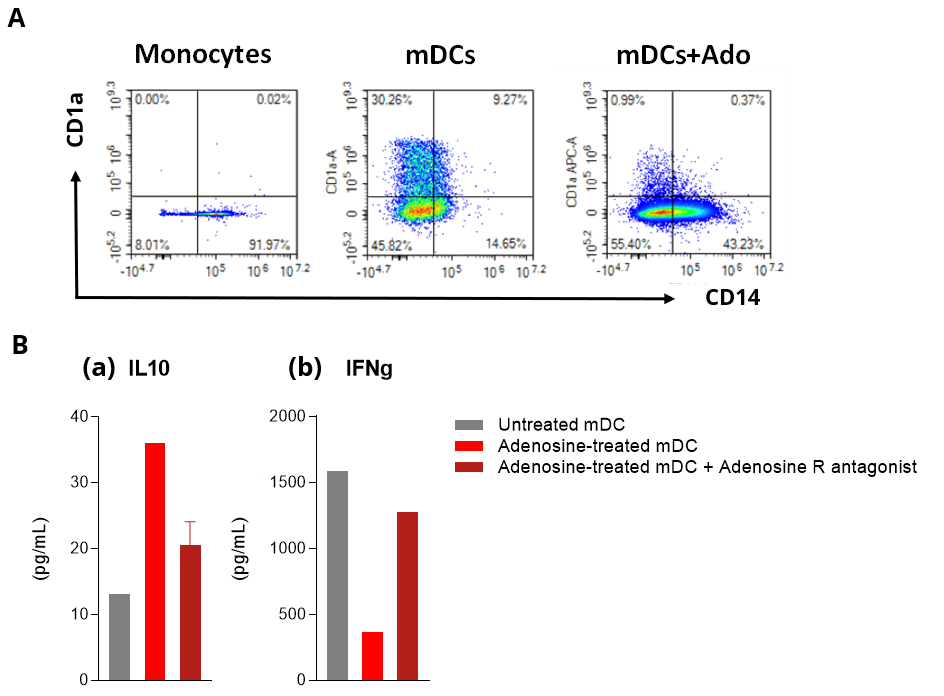
Adenosine impairs DC phenotype and function in allogenic human mDC/CD4 T cell co-cultures.
Isolated peripheral blood monocyte-derived DCs are challenged with Adenosine (Ado) in the presence and absence of an Adenosine Receptor (R) antagonist. Adenosine-mediated effect is then profiled by flow cytometry on DC differentiation and maturation (A), and supernatants from DC cultures or allogenic CD4+ T / DC co-cultures (B) are processed, respectively, for IL10 (a) and IFNg (b) HTRF quantification. (A) Adenosine is shown to skew DC differentiation and maturation toward a CD1a low CD14+ cell population, thereby inducing phenotypically dysregulated DCs. (B) While control mDC displays a MLR response – as expected, Adenosine strongly alters the allostimulatory ability of DCs to elicit T cell response, as shown through both the increase of IL10 levels released by DCs (a) and decrease of IFNg released by T cells (b). All these immunosuppressive effects are mediated through Adenosine receptors since relieved in the presence of an Adenosine R antagonist.
Why working with Explicyte?
Experts
in Immuno-Oncology
- Robust donor characterization, monocyte differentiation, and immunotherapy standards
- 100+ in vitro campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
Personalized
approach
- Targeted discussion based on your request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- A flexible technology platform to decipher your mechanism of action (immune checkpoint activation, signaling pathway, metabolic activity, …)
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
MLR assay to Test Novel Cancer Immunotherapies I CRO Services
The immunological synapse that occurs between T lymphocytes and Dendritic cells (DCs) is a key component of an effective immune response and is controlled by a combination of stimulatory and inhibitory signals – also called immune checkpoints. As to trigger a better anti-tumor immune response, interventions through a fine-tuning of these control signals within the immunological synapse represent one of the most promising cancer immunotherapy strategies. In order to investigate novel immunotherapeutics capable of enhancing DC-mediated T cell activation, Explicyte set up and validated a robust and high throughput human allogeneic mixed lymphocyte reaction (MLR) assay, which consists in a co-culture of CD4+ T cells (responder) with monocyte-derived DCs (stimulator) and in the HTRF-based quantitation of IFNγ, a relevant surrogate of T cell activation. Our assay is a well-suited system for biologics and small molecules assessment either as single agents and/or for combination therapies, including with e.g. immune checkpoint inhibitors, and can thus be ran in various types of studies spanning from screening to potency profiling and functional characterization of candidate compounds.