The underlying question: Does your compound modulate the proliferation & activation of immune cells and the release of cytokines?
- Format: 96-well plates
- Model: Primary immune cells (PBMCs or a subset such as T cells)
- Readouts:
- Immune cell proliferation by automated live-cell imaging
- Immune cell response via the capture of key cytokines (e.g. IFNg, TNFα, IL2 release)
- Standards: anti-PD1 and anti-PDL1 antibodies (Nivolumab, Pembrolizumab, and Atezolizumab)
- On request: flow cytometry immunophenotyping (e.g. immune checkpoint marker expression, activation marker expression…), cytokine array analysis, proteomics, transcriptomics
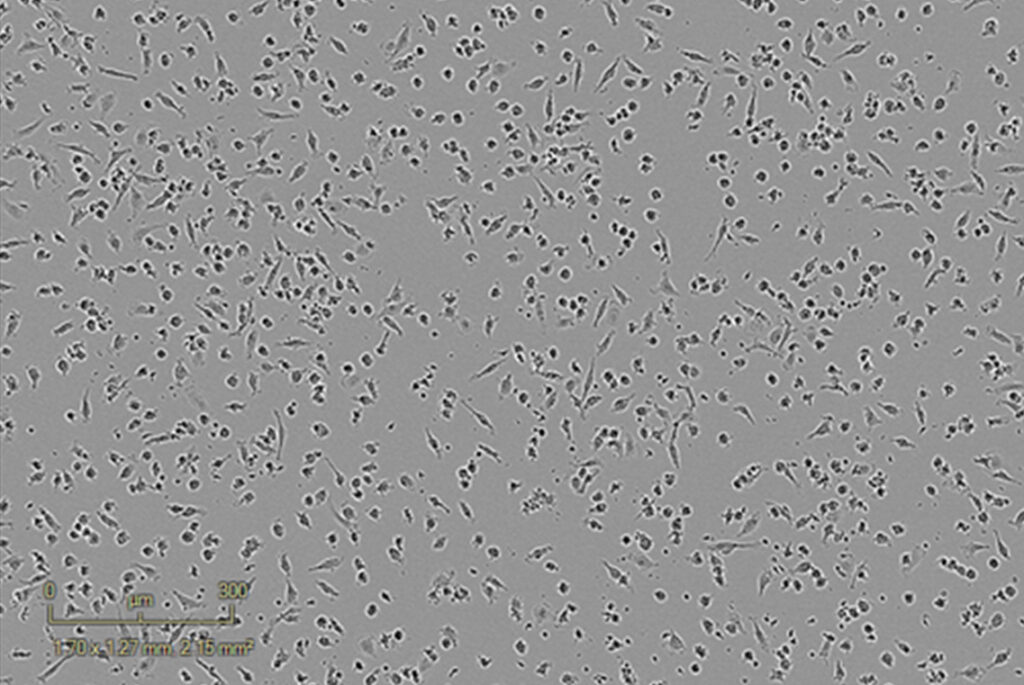
Inactivated PBMCs – Control
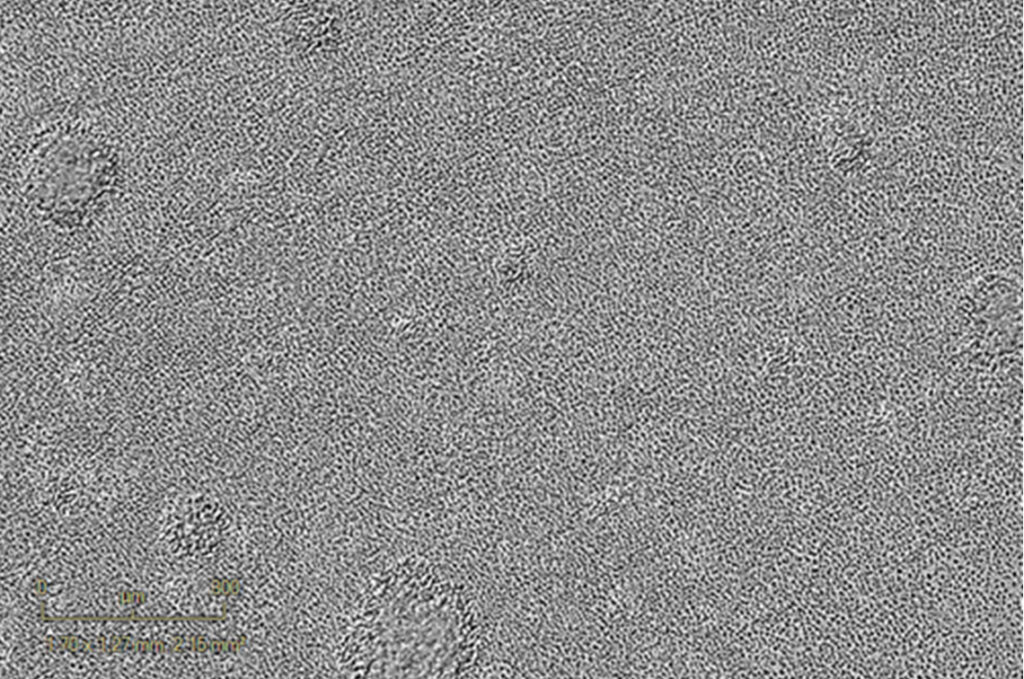
120h activated PBMCs (anti-CD3, 1µg/mL)
Assay principle
- PBMC or purified T cells are incubated with an activator cocktail (for TCR ligation, or any other immune stimulus )
- Activated immune cell proliferation is visualized and measured over time by live cell imaging
- Immune cytokine release is quantified by HTRF or LEGENDplex™
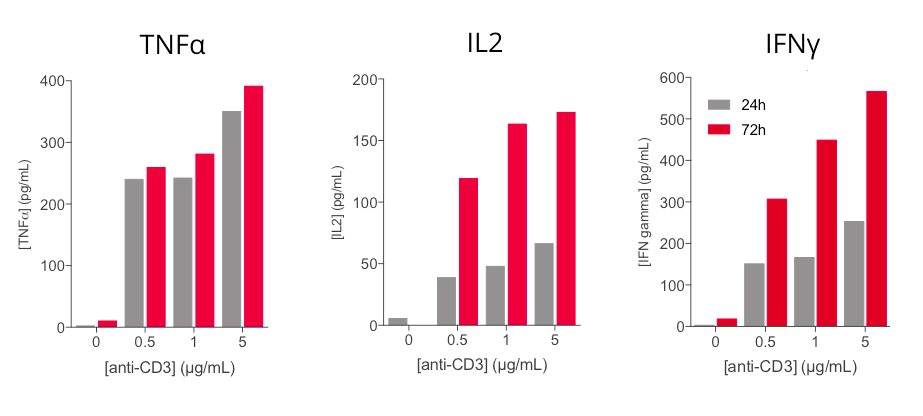
Anti-CD3 based PBMC activation induces a dose- and time- dependent release of key inflammatory cytokines
Key inflammatory cytokines i.e. TNFα, IL2, and IFNγ are shown to be modulated upon human PBMC stimulation with increasing concentrations of anti-CD3 antibody. While TNFα secretion is efficiently induced at 24h, released IFNg and IL2 levels are time- and dose-dependently increased following anti-CD3 treatment. These cytokines, and in particular IL2 and IFNg, appear as valuable surrogates to ascertain PBMC activation.
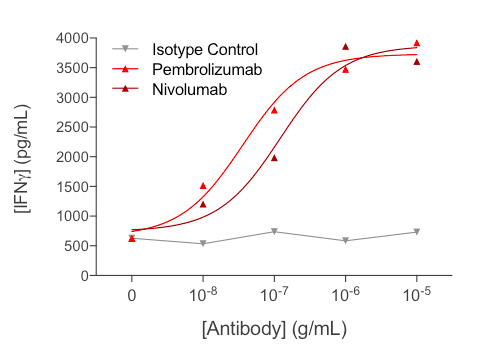
Anti-CD3-induced IFNγ released by human PBMCs is optimized by reference anti-PD1 antibodies
Treatment of anti-CD3-activated PBMCs with increasing concentrations of each of anti-PD1 antibodies (Nivolumab or Pembrolizumab) leads to a significant dose-dependent increase of IFNγ release, thereby reflecting further optimization of PBMC activation.
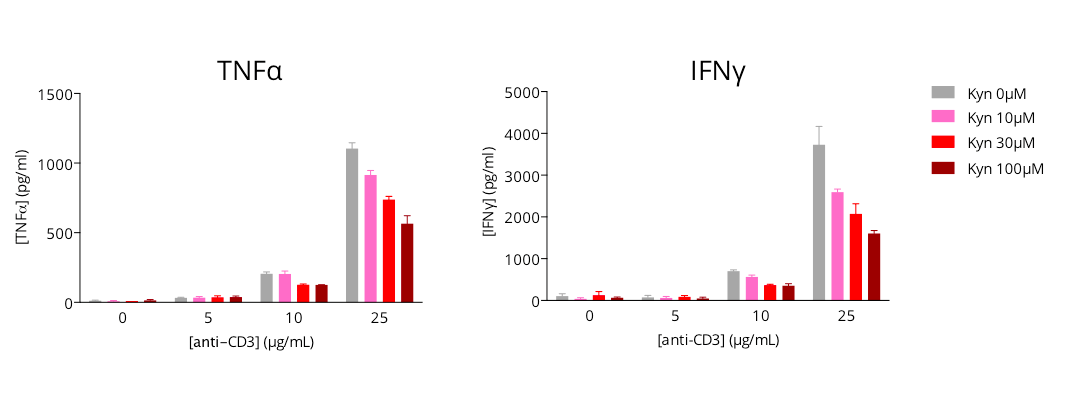
L-Kynurenine-mediated immunosuppression of activated T cell response
Human primary CD4+ T cells are modulated in a dose-dependent manner upon anti-CD3 treatment, as illustrated by the production of key inflammatory cytokines – TNFα and IFNg, quantified by HTRF 72h post- culture. Concomitant L-kynurenine addition with anti-CD3 is shown to dose-dependently limit the anti-CD3-induced T cell activation, thus highlighting the immunosuppressive action of L-kynurenine. Therefore, assessing cytokine release upon T cell activation represents a valuable approach for the evaluation of immunomodulatory candidates.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 100+ in vitro campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
Personalized
approach
- Targeted discussion based on sponsor request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- A flexible technology platform for in-depth mechanism-of-action studies
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
Immune Cell Activations Assays I Immuno-Oncology CRO services
Modulation of the immune system function aiming at enhancing the potential of immune cell responsiveness is one promising challenge for cancer therapy. During the immune response, T cells become activated and undergo clonal expansion followed by differentiation into effector cells and induction of cell-mediated cytotoxicity and/or cytokine release. Understanding and modulating the mechanisms underlying activation, proliferation & clustering of immune cells is key for the development of innovative approaches to promote immune cell function and tackle tumor progression. One of the most common ways to measure immune cell activation in vitro is the assessment of T cell proliferation upon antigen- or TCR-mediated stimulation. In this respect, Explicyte is offering valuable in vitro PBMC- and T cell-based 96-w plate assays to evaluate the effects of immunomodulatory compounds/antibodies alone and in combination with anti-CD3 antibody, on immune cell activation. By live cell time-lapse imaging, treatment effects are evaluated in a kinetic and phenotypic manner on the immune cell proliferation and clustering, as well as functionally on the release of key effector cytokines.










