Flexible sampling, ranging from cell cultures to clinical specimens
- Liquid samples: including cell cultures, preclinical, and clinical blood samples, which can be generated in-house, acquired, or provided by the sponsor.
- Tissue homogenates: in-house preparation of preclinical ex vivo samples (tumor and organs, e.g., spleen) and patient-derived tumor specimens.

Multiplexed immunophenotyping & anti-tumor immune response profiling
- Agilent NovoCyte Quanteon flow cytometer: capable of quantifying up to 17 parameters with validated & customized marker panels to track the efficacy and mechanisms of action of cancer immunotherapies.
- Comprehensive panel design: we provide expertise in designing and optimizing multiparametric flow cytometry panels to analyze various cell populations and biomarkers
Advanced data analysis & visualization
- A dedicated study director with expertise in gating strategy (FMO/isotype-based).
- Routine FlowJo Analysis combined with our internal analysis pipeline.
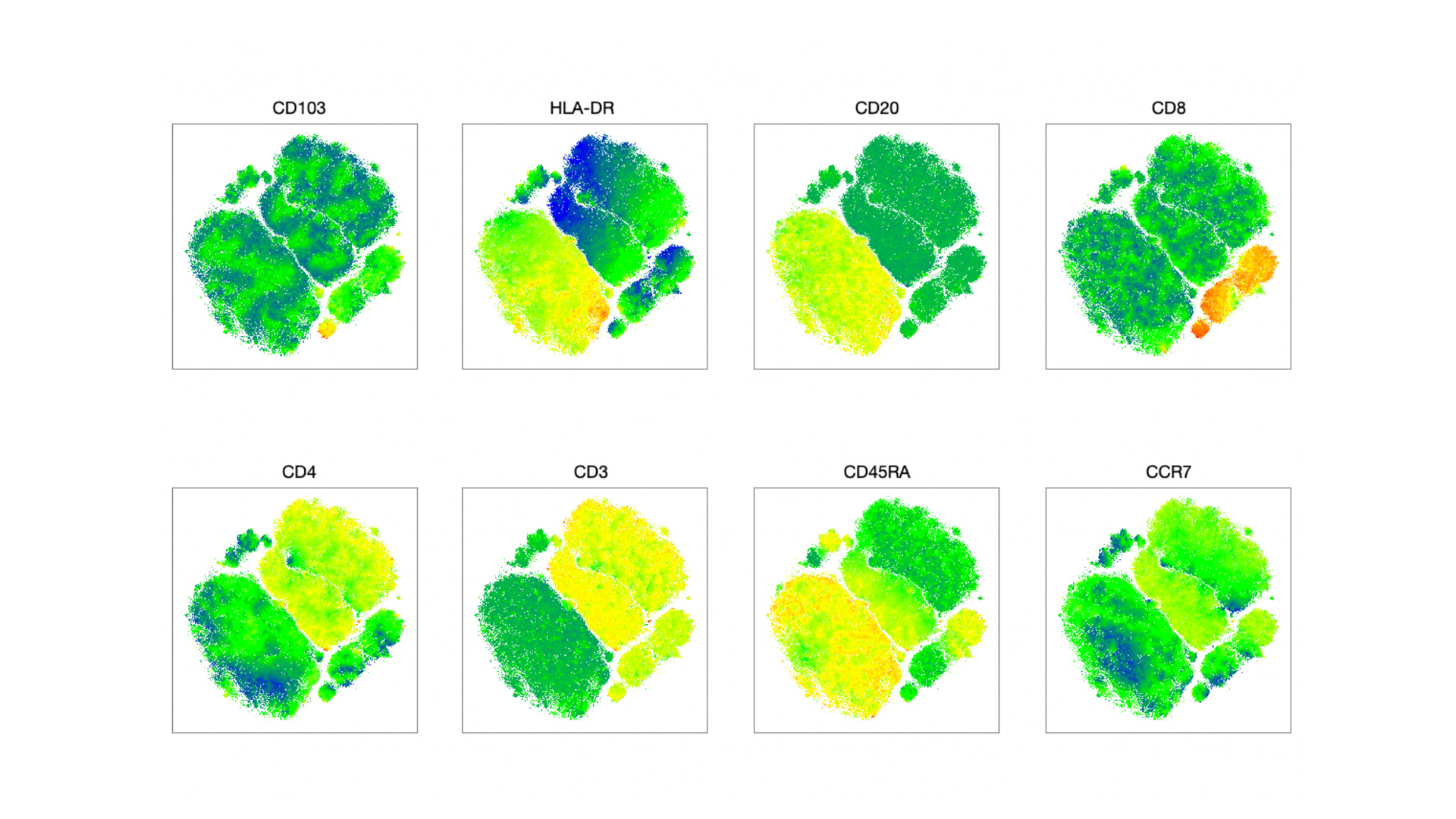
- tSNE Methodology to visualize high-dimensional data.
Experts
in Immuno-Oncology
- Strong expertise in flow cytometry: over 1,000 samples analyzed per year since 2015
- Experience with a wide range of markers linked with anti-tumor immune response
- 30+ peer-reviewed publications in key immuno-oncology journals
Personalized
approach
- A dedicated study director (PhD) to fit the panel design and analysis plan to your need
- A panel of validated markers, which can be completed with custom markers
- Complementary technology platforms: cytokine analysis by HTRF, proteomics, single-cell RNA sequencing, digital pathology, spatial transcriptomics, …
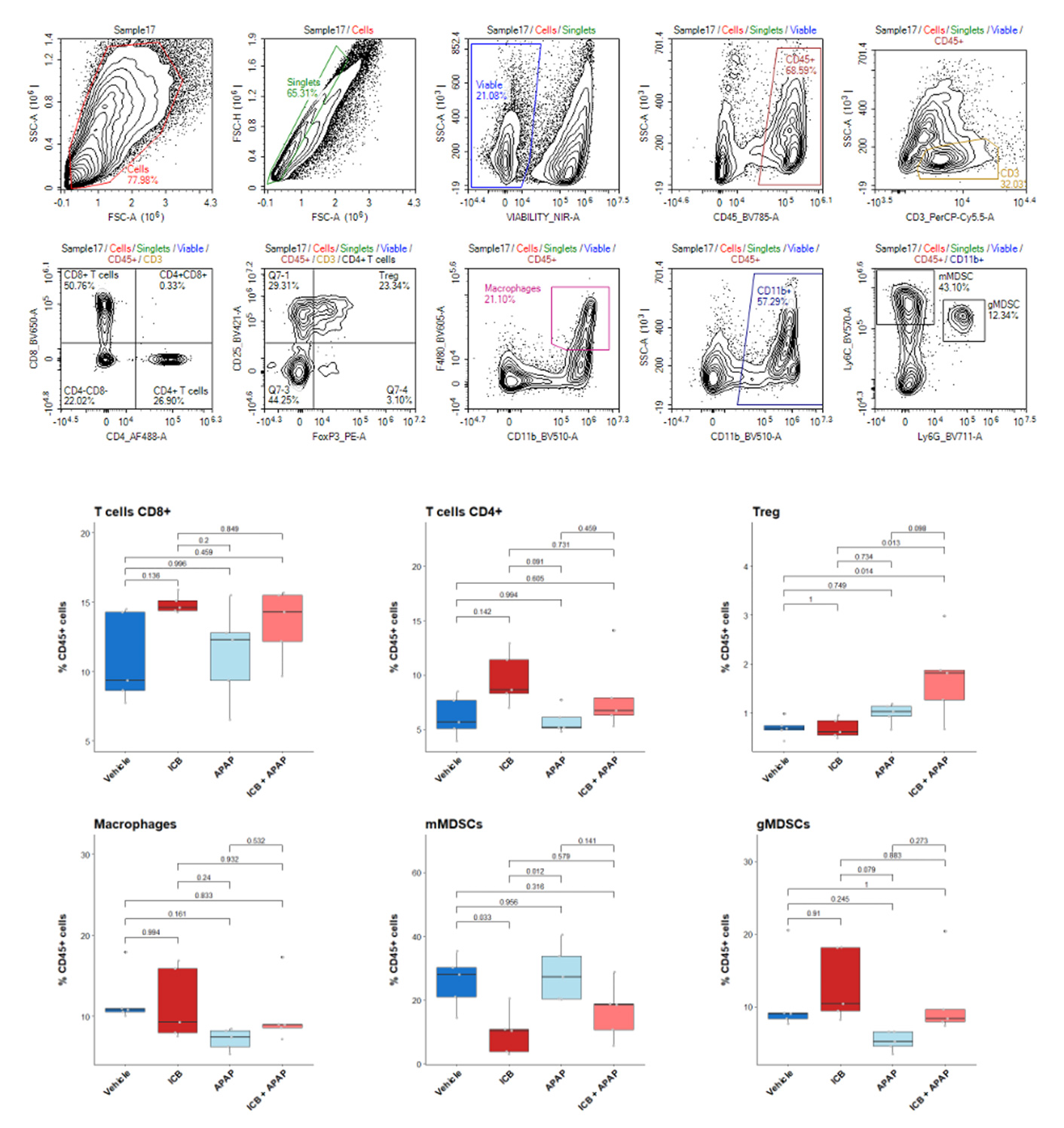
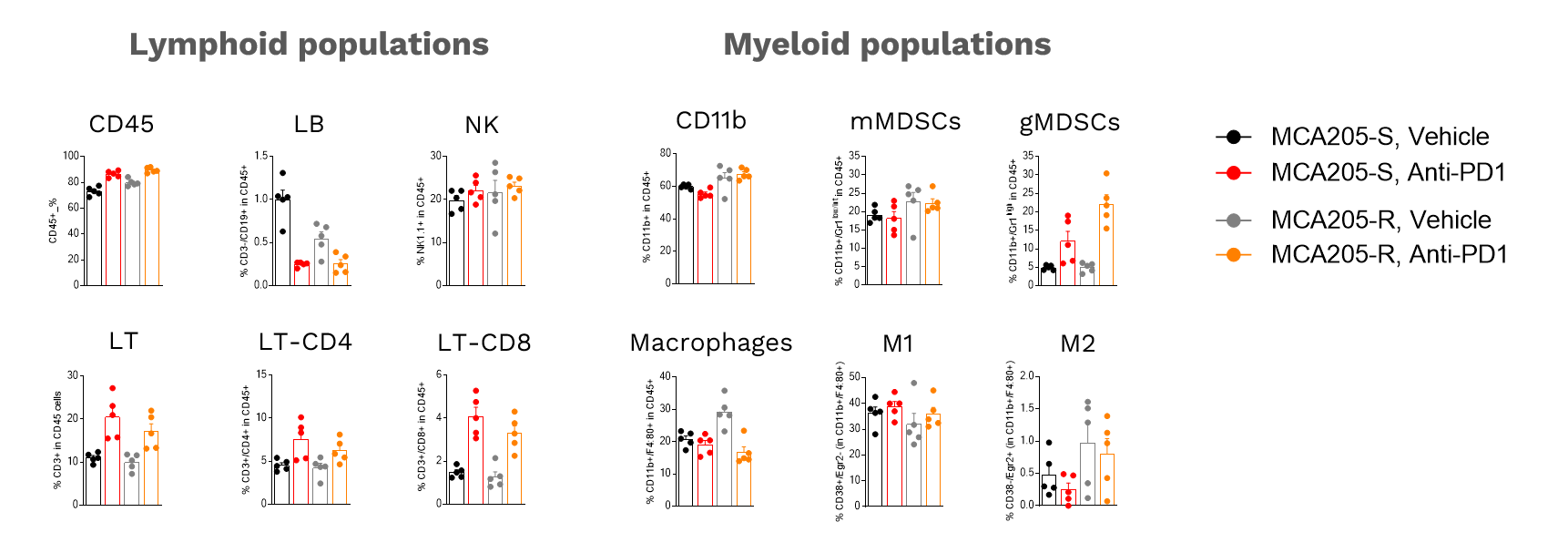
Profiling of MCA205 tumor-infiltrating leukocytes upon anti-PD1 immunotherapy
Flow cytometry Analysis of anti-PD1 MCA205-S and MCA205-R tumor immune cell infiltrate, upon vehicle (n=5) or anti-PD1 antibody (n=5) treatments. Tumors were retrieved on day 16 post-tumor inoculation and processed for immunostaining and analysis by multiparametric flow cytometry of the Lymphoid and Myeloid components, according to a multiplexed marker panel including CD45, CD3, CD4, CD8, CD19, NK1.1, CD11b, F4:80, Gr1, CD38, Egr2, and viability markers.
In collaboration with Institut Gustave Roussy and Institut Bergonié, Explicyte has demonstrated the detrimental role of acetaminophen (APAP)—one of the most widely used medications worldwide—on the response to immunotherapy. These findings are supported by both clinical evidence and preclinical testing showing a higher abundance of regulatory T cells within the tumor upon APAP exposure. These results are published in Annals of Oncology.
Mice bearing MC38 colon tumors were treated or not with acetaminophen from days 5 to 22 (30mg/kg – oral gavage) together with anti-PD-(L)1 antibodies on days 6, 9, 12, and 15 (5mg/kg , i.p.). Tumor biopsies (n=5 per group) were collected on Day 13 post tumor inoculation and infiltrating cells were identified by flow cytometry – workflow of the gating strategy of mouse tumor panel is highlighted on top. P value was calculated using ANOVA test with Tuckey. mMDSCs (monocytic Myeloid Derived Suppressor Cells), gMDSCs (granulocytic Myeloid Derived Suppressor Cells).
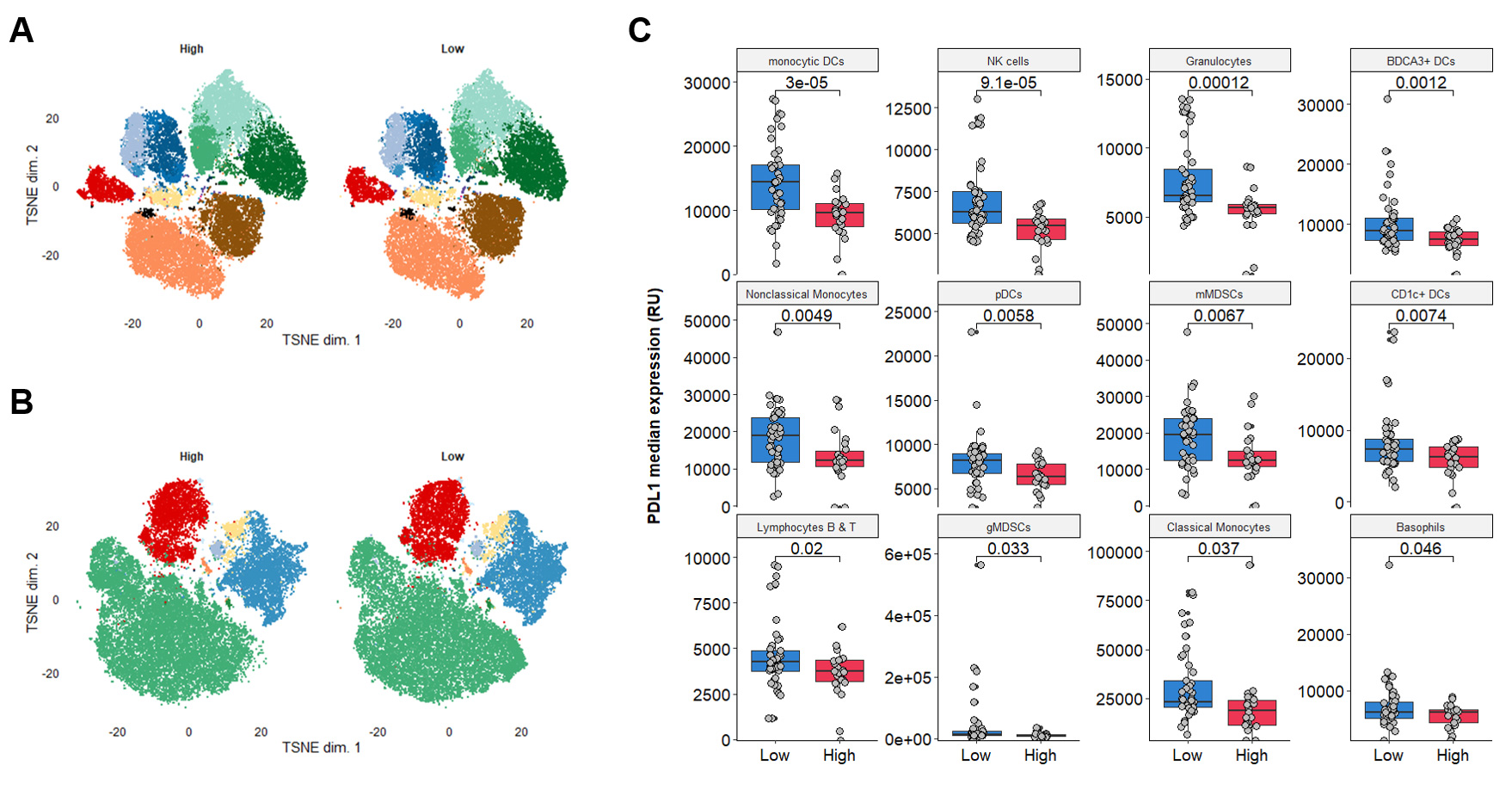
Explicyte, in collaboration with the Gustave Roussy and Institut Bergonié Comprehensive Cancer Centers, presented a poster at the 2022 AACR annual meeting demonstrating the association between plasma arginine levels and response to immune checkpoint inhibitors. In this study, thanks to extensive flow cytometry analysis, the authors highlighted the association between arginine levels and PDL1 expression by different circulating immune cells. These results were published in Annals of Oncology.
Multiplexed flow cytometry analysis of matched PBMC-plasma samples to explore the correlation between Arg level and PBMCs features
A-B. t-SNE visualizations of cluster assignments of PBMCs from arginine High and Low patients treated with ICI. PBMCs were stained with the lymphoid panel (A) and myeloid panel (B). No differences in cell population composition were noted between patients. C. Quantification of PDL1 expression (MFI) in the different cell populations identified within the myeloid panel.
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
Flow Cytometry Services for Immuno-Oncology I CRO Cancer Immunotherapy
In addition to its relevant flow cytometry platform, explicyte has extensive know-how and expertise for the multiplexed detection and analysis of markers of interest. Analyzing series of specific biomarkers or markers (intracellular, surface, or secreted) provides indeed a thorough insight into cellular function, thereby enabling the characterization of pathology-underlying processed or of mechanisms of action of drug candidates. Also, we provide flow cytometry-based services as gold standard for immunophenotyping and immune response profiling, for which both standardized and customized marker panels are performed, so as to meet our specific clients’ needs and support their drug discovery & screening programs as well as biomarker expression and pharmacokinetic assessment. Key features and advantages of flow cytometry: The gold-standard for immunophenotyping and immune response profiling: marker analysis in specific cell subsets / Well-suited for multiplexed detection of markers of interest (intracellular, surface markers, phosphoproteins) and compatible with high throughput / Appropriate for multiplexed detection of secreted biomarker molecules e.g. cytokines in a multitude of biological samples such as supernatants, blood derivatives, and tissue homogenates).