-
- Model features: metastatic breast carcinoma, immunosuppressive microenvironment, privileged immunometabolic profile
- Tumor cell line: EMT6 murine mammary carcinoma cells
- Tumor implantation: subcutaneous or orthotopic (mammary fat pad)
- Standards: immune checkpoint inhibitors (anti-CTLA4, PD1 antibodies), cisplatin
- Readouts: body weight, tumor size, survival
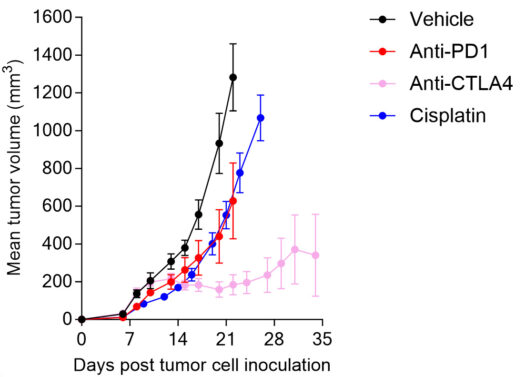
Anti-tumor response of orthotopic breast EMT6 tumor model to cisplatin and immune checkpoint blockers (anti-PD1 and anti-CTLA4 antibodies).
In vivo efficacy & mechanism of action studies for novel immunotherapies
Straightforward in vivo efficacy studies
- N=10: Standard groups of 10 mice including groups exposed to test compound alone and in combination with reference therapy.
- Weekly reports: monitoring tumor growth, body weight, and survival
Flexible sampling options
- Monitoring response over time: satellite mice, serial bleeding, intra-tumoral biopsies
- On-demand sample collection: blood, serum, plasma, tumor, organ samples
A flexible platform to quantify tumor-microenvironment & peripheral markers
- Multiplex immunophenotyping by flow cytometry & digital pathology
- Spatial transcriptomics & proteomics
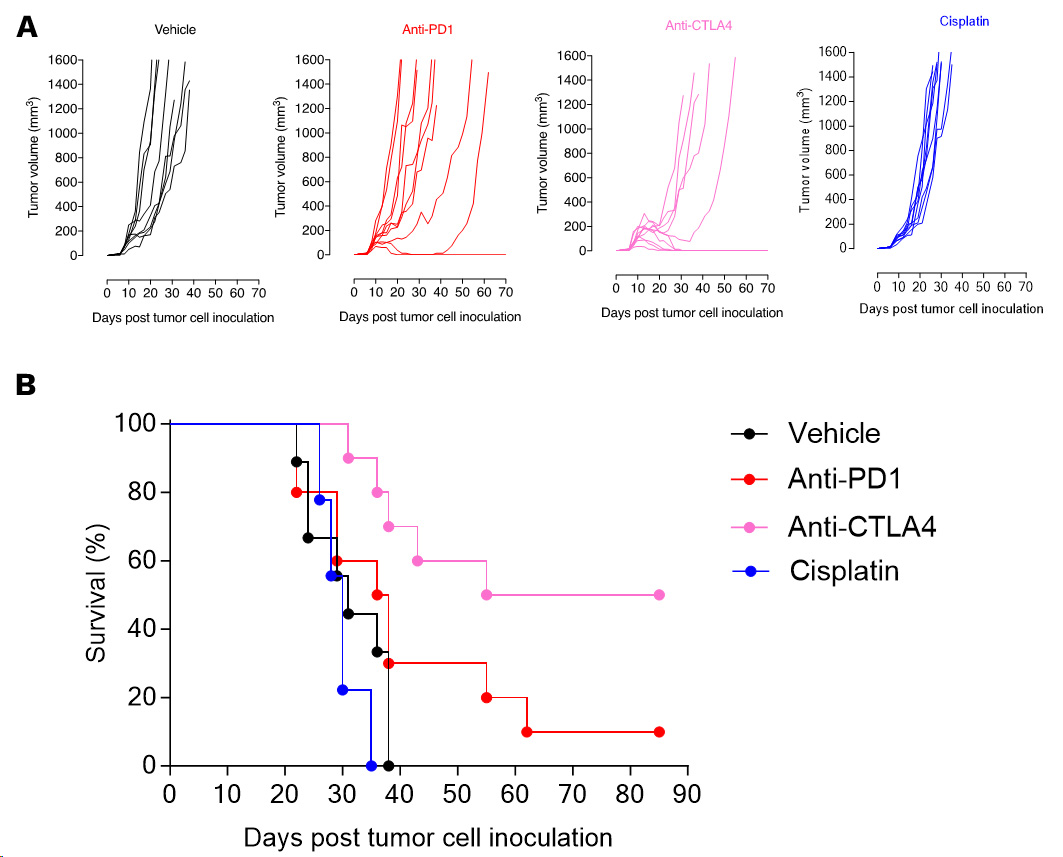
Orthotopic breast EMT6 tumor model, cisplatin-insensitive, is responsive to immune checkpoint inhibitors.
Individual tumor volume (mm3) (A) and Kaplan-Meier plot survival (B) of EMT6 tumor-bearing mice upon vehicle, anti-PD1, anti-CTLA4 antibody, or cisplatin treatments.
While EMT6 tumors are insensitive to cisplatin chemotherapy, it displays sensitivity to immune checkpoint inhibitors – being moderately responsive to CTLA4 and PD1 blockade (with a stronger response to anti-CTLA4), the model still offers enough range for potential improvement upon combinatorial strategies.
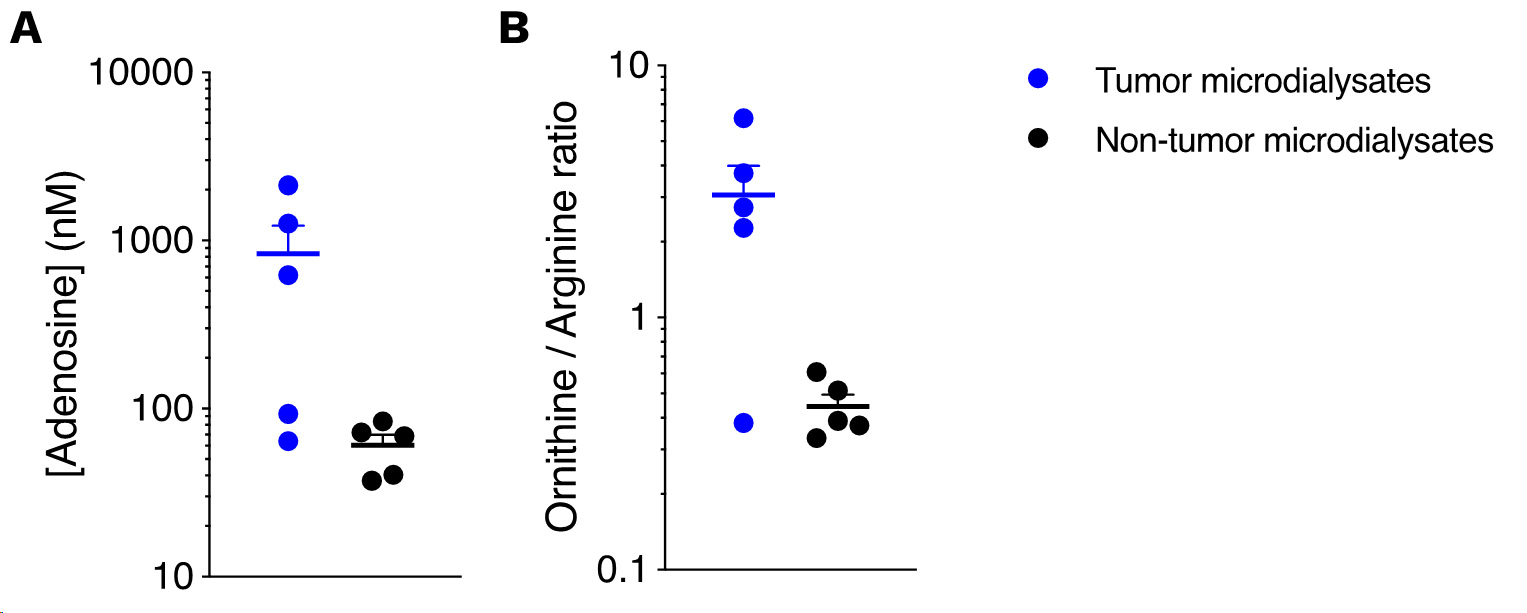
Immunometabolic profiling of the orthotopic EMT6 breast model by intratumoral microdialysis.
Mice were implanted with tumor cells and then processed for intratumoral microdialysis at the tumor volume average of ~200mm3, for the determination of adenosine (A), arginine and ornithine (B) levels by LC/MS. While adenosine and arginine pathways are both known to be involved in the immune escape, the tumor model displays high levels for these metabolites, thereby conferring it a peculiar immunometabolic profile to study and alleviate immunosuppression.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 150+ in vivo campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- Bespoke study designs based on client objectives and literature
Personalized
approach
- A dedicated study director (PhD level) from experimental plan to final report
- Weekly reports to provide regular updates & adapt experimental strategy
- Comprehensive analytical platform to decipher anti-tumor response
Your scientific contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
EMT6 Syngeneic Breast Cancer Murine Model I in vivo CRO Services in Immuno-Oncology
Because of metastatic development of the disease, among other characteristics, and regardless of survival data, breast cancer was shown to be particularly difficult to treat. However, metastases as well as resistance to therapies continue to be a great challenge and the development of new therapeutic strategies is increasingly necessary. Preclinical CRO Explicyte Immmuno-Oncology has fully characterized a syngeneic orthotopic EMT6 breast tumor mouse model. While immunometabolic profiling is suggestive of an immunosuppressive microenvironment, EMT6 model is only moderately responsive to immune checkpoint blockade and thus still remains offering enough range to gain improvements in combinatorial strategies, for instance. Hence this model is featured of a favorable immune profile thereby making it a powerful preclinical immuno-oncology tool to be employed in investigating novel treatment combinations.










