The underlying question: Does your compound cause cytokine release syndrome (CRS), and could it trigger an excessive immune response?
- Format: 96-well plates
- Primary immune cells: human PBMCs from multiple donors
- Multiplex cytokine analysis: Up to 13 cytokines, based on LegendPlex™, measured at 6h and 24h after exposure to the test compound
- Dose-response: test compound is introduced at escalating doses (soluble and immobilized formats can be used)
- TGN1412 as standard: immobilized superagonist anti-CD28 mAb has triggered life-threatening CRS in first-in-human trials
- On request: In case of immune activation, integration of immunophenotyping, gene expression, or proteomics to investigate the underlying mechanisms of action
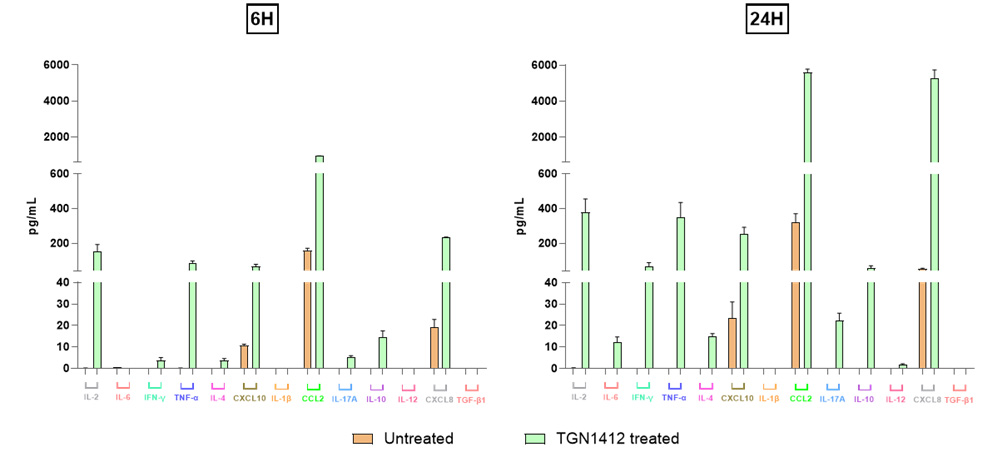
Quantification of 13 Cytokines in Untreated vs. TGN1412-treated PBMCs at 6h and 24h
This figure displays the quantification of 13 key cytokines involved in immune responses, measured in the supernatant of PBMC cultures. Cytokine levels were analyzed in untreated and TGN1412-treated samples at two time points: 6 hours and 24 hours post-treatment. Each bar represents the concentration (pg/mL) of a specific cytokine, with comparisons shown between untreated and treated samples for each time point. Error bars indicate the standard deviation based on replicate samples. The cytokine panel includes IL-2, IL-6, IFN-γ, TNF-α, IL-4, CXCL10 (IP-10), IL-1β, CCL2, IL-17A, IL-10, IL-12p70, CXCL8 and TGF-β1.
Assay principle
- PBMC exposure to test compound
- At 6- & 24-hours post-treatment: quantification of 13 key cytokines (IL-2, IL-6, IFN-γ, TNF-α, IL-4, CXCL10 (IP-10), IL-1β, CCL2, IL-17A, IL-10, IL-12p70, CXCL8 and TGF-β1) by LegendPlex
- Additional assays & readouts: combination with cell proliferation and cytotoxicity assay
Why working with Explicyte?
Experts
in Immuno-Oncology
- 100+ in vitro campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- A comprehensive technology platform to decipher mechanisms behind cytokine release profiles
Personalized
approach
- Targeted discussion based on your request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- Custom secondary analysis to understand the cytokine release profile highlighted by the assay and assess the safety profile of your compound
Tell us about your project !

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Cytokine Release Assay I Cytokine Storm I Cytokine Release Syndrome I CRO services
In the development of cancer immunotherapies, understanding and mitigating potential immune overactivation is critical. One such severe immune response is a cytokine storm, an uncontrolled release of cytokines that can lead to systemic inflammation and severe patient outcomes. Cytokine storms are particularly relevant in immunotherapy because many immune-modulating treatments can potentially trigger these dangerous reactions. To assess the safety profile of new compounds, Explicyte has developed a cutting-edge in vitro cytokine storm assay. This assay uses Peripheral Blood Mononuclear Cells (PBMCs) cultured with the test compound, and after 6 and 24 hours, cytokine levels in the supernatant are measured using LegendPlex technology to analyze a panel of 13 key immune response cytokines: IL-2, IL-6, IFN-γ, TNF-α, IL-4, CXCL10 (IP-10), IL-1β, CCL2, IL-17A, IL-10, IL-12p70, CXCL8 and TGF-β1. This broad cytokine profile enables a comprehensive assessment of potential immunomodulatory effects, ranging from pro-inflammatory to anti-inflammatory responses. An important aspect of this assay is the use of TGN1412 as a positive control. TGN1412 is a monoclonal antibody that notoriously triggered a life-threatening cytokine storm in its first human trial, an outcome that could have been anticipated and mitigated with an in vitro cytokine storm assay like ours during its preclinical phase. By using TGN1412 as a control, we can verify that our assay is sensitive to cytokine storm-inducing compounds, providing a reliable model to screen new immunotherapeutic agents for their safety and cytokine release profiles. This test is essential for identifying compounds that may pose a risk of excessive immune activation, enabling safer development of immunotherapies, especially those targeting immune checkpoint pathways, T-cell activation, and other potent immune responses.










