-
- Model features: aggressive breast carcinoma with spontaneous metastasis, immune-infiltrated, immunosuppressive microenvironment
- Tumor cell line: 4T1 breast carcinoma cells (Luc-2-expressing cells available for in vivo imaging)
- Tumor implantation: subcutaneous or orthotopic implantation in mammary fat pad
- Standards: Doxorubicin, cisplatin, immune checkpoint inhibitors (anti-PD1, anti-PDL1, anti-CTLA4)
- Readouts: body weight, tumor size, survival
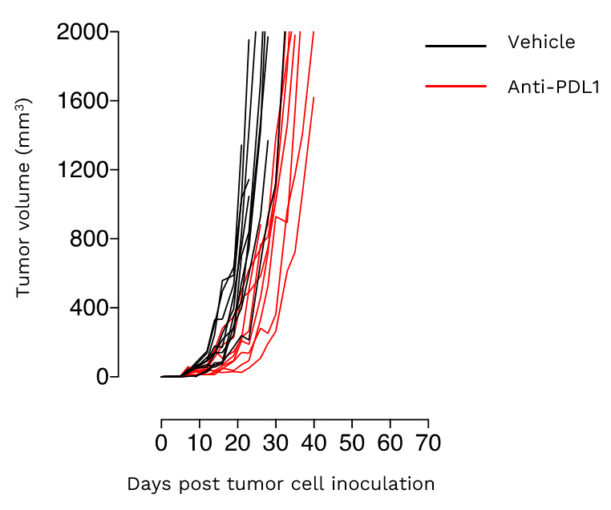
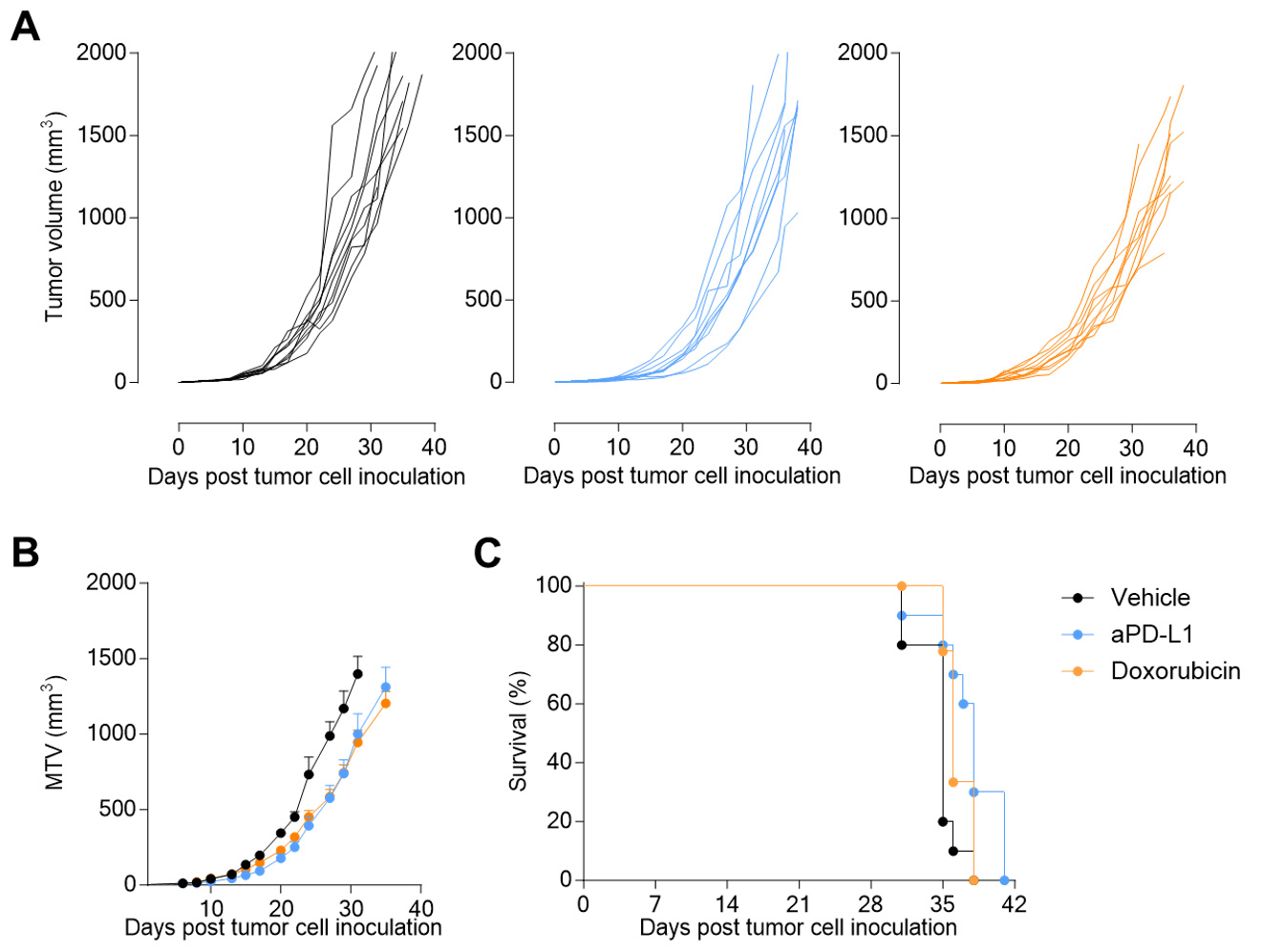
Subcutaneous 4T1 breast tumor model exhibits a weak sensitivity to PDL1 inhibition.
Mice were challenged with 4T1 cells and exposed to vehicle or anti-PDL1 antibody. Tumor growth was monitored overtime.
In vivo efficacy & mechanism of action studies for novel immunotherapies
Straightforward in vivo efficacy studies
- N=10: Standard groups of 10 mice including groups exposed to test compound alone and in combination with reference therapy.
- Weekly reports: monitoring tumor growth, body weight, and survival
Flexible sampling options
- Monitoring response over time: satellite mice, serial bleeding, intra-tumoral biopsies
- On-demand sample collection: blood, serum, plasma, tumor, organ samples
A flexible platform to quantify tumor-microenvironment & peripheral markers
- Multiplex immunophenotyping by flow cytometry & digital pathology
- Spatial transcriptomics & proteomics
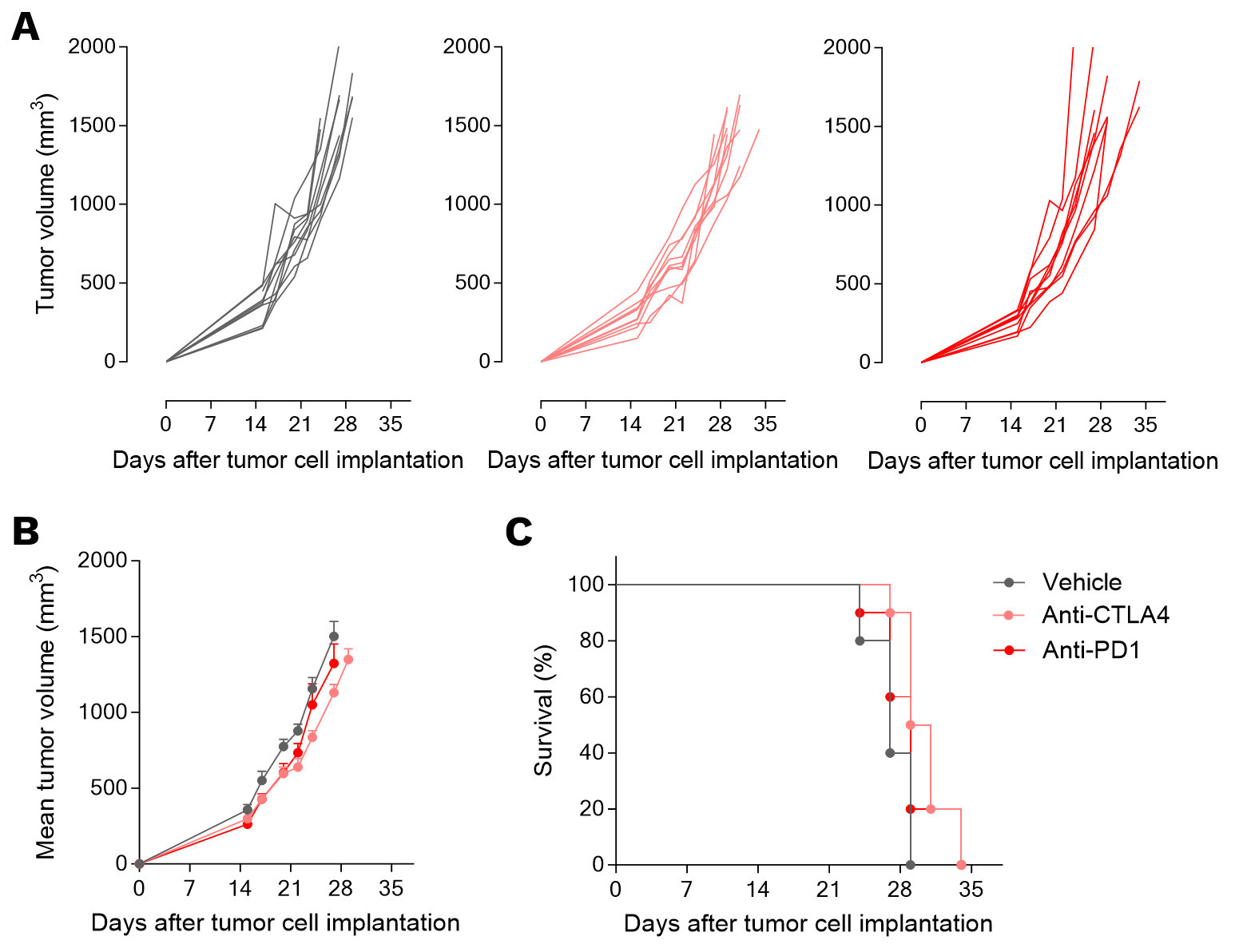
Orthotopically-implanted 4T1 breast tumor-bearing mouse model is non-responsive to conventional immunotherapy.
Mice were OT implanted with 4T1 murine breast tumor cells and exposed to anti-PD1 or anti-CTLA4 antibody. Tumor volume (A, B) and survival (C) were monitored overtime.
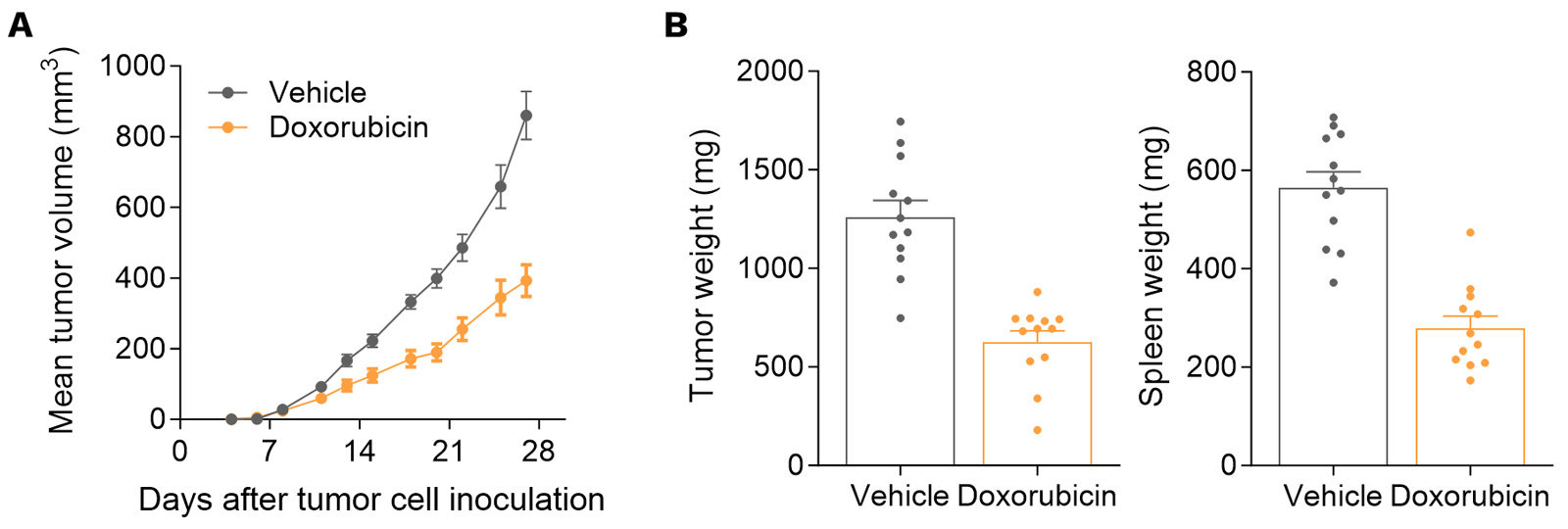
Orthotopic syngeneic 4T1 mammary fat pad model is moderately doxorubicin-sensitive.
Mice were OT implanted with 4T1 murine breast tumor cells and treated with doxorubicin. Tumor growth was monitored overtime in vivo via calipering (A). At necropsy, tumor and spleen weights were determined (B).
Doxorubicin triggers a decrease in tumor growth.
PDL1 blockade and doxorubicin trigger weak anti-tumor effects in the subcutaneously-implanted 4T1 breast tumor-bearing mice.
Tumor growth (A, B) and survival (C) were monitored overtime.
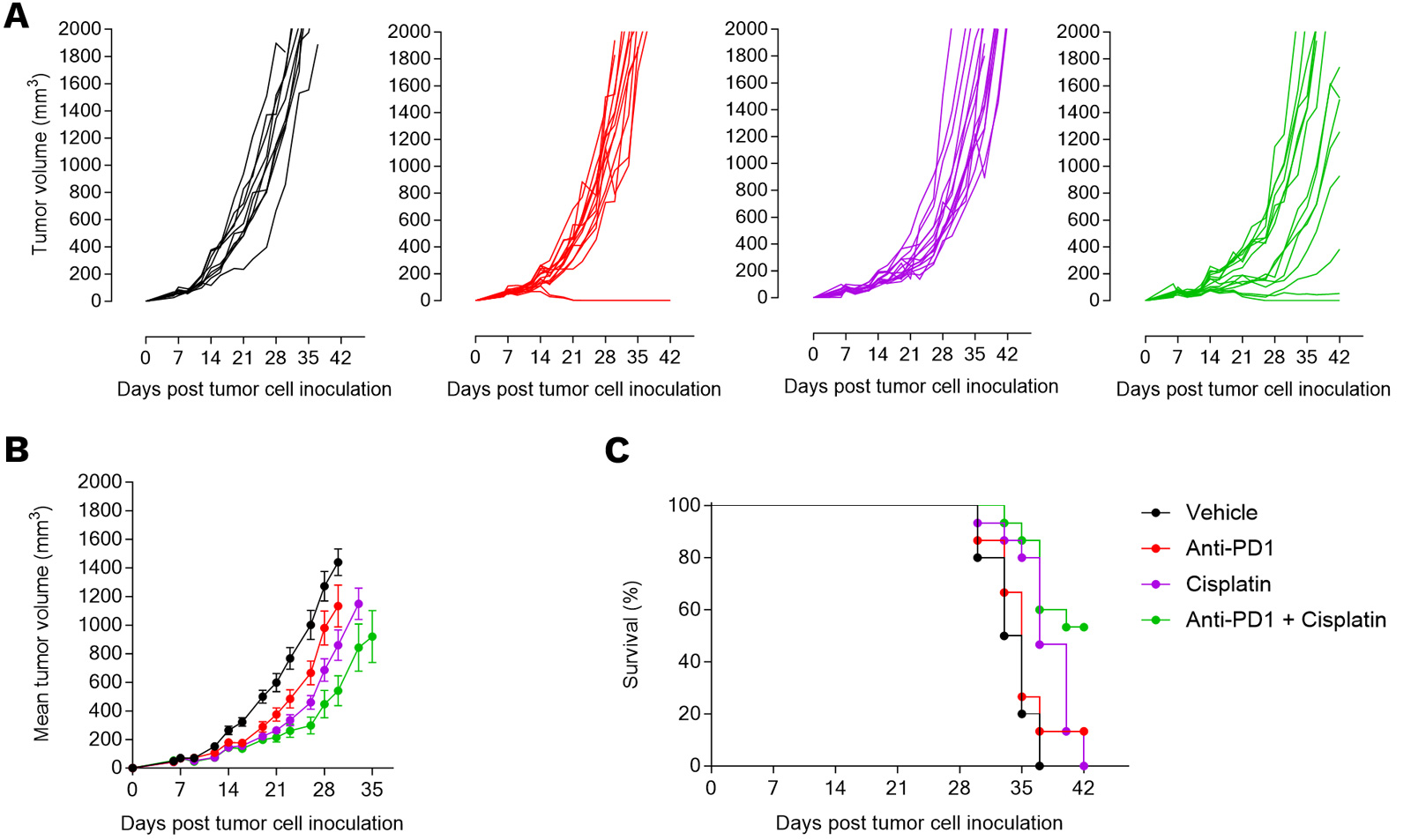
PD1 blockade and cisplatin each elicit a weak-to-moderate anti-tumor response in the subcutaneously-implanted 4T1 tumor model, which is further optimized when combining the two agents.
Mice were implanted with 4T1 murine breast tumor cells and exposed to anti-PD1 antibody, cisplatin, or combination. Tumor growth (A, B) and survival (C) were monitored overtime.
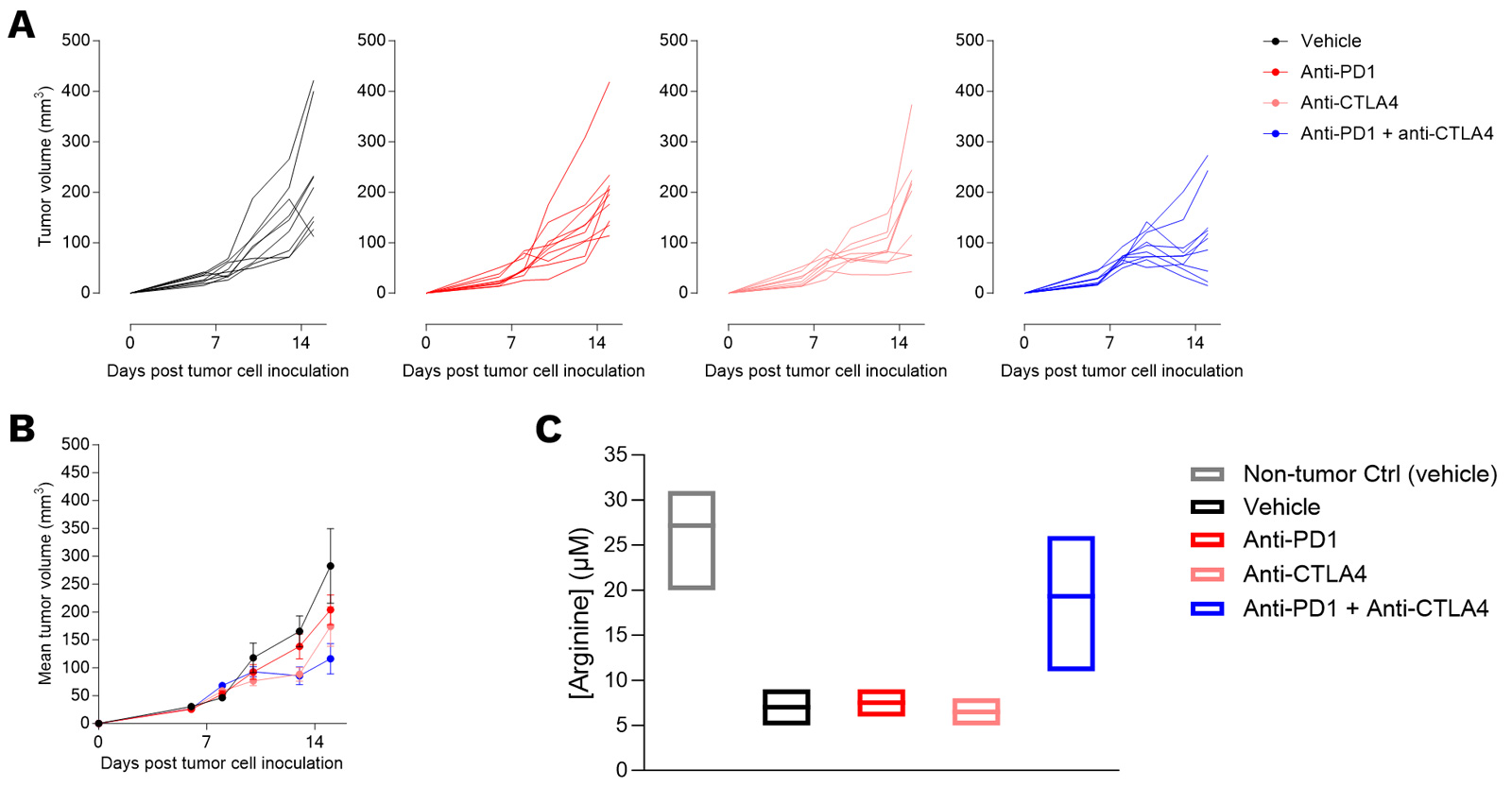
Subcutaneous 4T1 breast tumor model exhibits an optimized sensitivity to conventional immunotherapy upon CTLA4 and PD1 blockade combination, underpinned by a limitation of arginine degradation.
Mice were implanted with 4T1 murine breast tumor cells and exposed to anti-PD1 or anti-CTLA4 antibodies, alone or combination. Tumor growth (A, B) and survival (C) were monitored overtime up to the analysis timepoint for microdialysis. At the analysis timepoint, mice were processed for tumor microdialysis (and non-tumor, for on the contralateral side to the vehicle tumors) and ELISA quantification of arginine (C).
Why working with Explicyte?
Experts
in Immuno-Oncology
- 150+ in vivo campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- Bespoke study designs based on client objectives and literature
Personalized
approach
- A dedicated study director (PhD level) from experimental plan to final report
- Weekly reports to provide regular updates & adapt experimental strategy
- Comprehensive analytical platform to decipher anti-tumor response
Your scientific contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Tell us about your project !
4T1 Syngeneic Mouse Tumor Model I Cancer Immunotherapy in vivo services I Preclinical CRO
Based on surgical breast tumor cell inoculation into the fourth mammary fat pad of immunocompetent mice, our syngeneic orthotopic 4T1 breast tumor-bearing mouse model, which is an aggressive model of human breast carcinoma, has indeed many characteristics that make it a suitable experimental tool for human breast cancer. In addition to their poor immunogenicity – a characteristic shared with human mammary cancers, 4T1 tumor cells are easily implanted into the mammary fat pad thereby allowing for the growth of the primary tumor in the anatomically correct site. Moreover, 4T1 model is capable of spontaneous metastasis to several organs including and not limited to lungs and liver, in a pattern that is analogous to human mammary cancer. Metastases begin while the primary tumor is in place but are heterogenous and not detectable in all animals.
In an interesting way, the model is characterized by the presence of at least two types of tumor-infiltrating immunosuppressive cells – myeloid suppressive cells (MSC) and T regulatory cells (Treg). Such cell populations are known, as a result, to suppress T cell responses and thus promote tumor immune escape and metastasis. Our orthotopic syngeneic breast tumor model is a well-suited tool for preclinical testing of new anti-cancer therapies that require an intact immune system to elicit activity, e.g. checkpoint inhibitors or targeted therapies, which would directly target cancer cells or aim at suppressing the immunosuppressive cell functions. Interestingly, our model is run with stable Luc-2-expressing 4T1 tumor cells which has the advantage to monitor tumor growth by in vivo bioluminescence imaging (BLI).