Target expression analysis
In patient tumor specimens or tumor cell lines to identify and validate relevant indications and biological systems of interest.
ADC binding & specificity
Assessment using flow cytometry and/or ELISA
ADC internalization
Evaluation by flow cytometry or live cell time-lapse imaging
ADC cytotoxicity profiling & efficacy
In functional tumor cell killing assays and 3D patient-derived tumor models
ADC safety analysis
in vitro/in vivo analysis of ADC immunogenicity and off-target effects
Biomarker analysis
Exploration of biomarker expression in preclinical and clinical samples
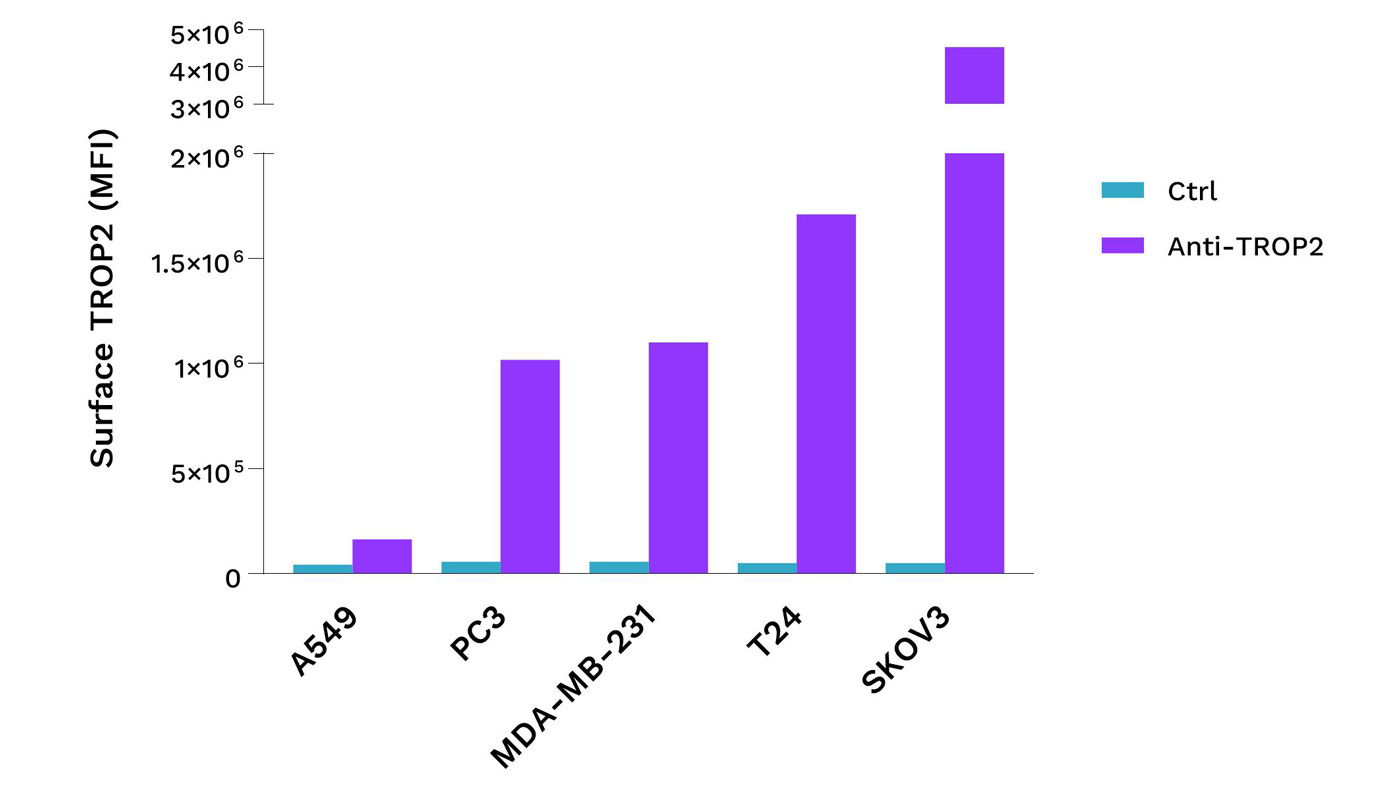
Selection of cell lines based on TROP2 expression
TROP2 is expressed in cell lines of many cancer indications. Flow cytometry analysis of TROP2 expression was performed in various cancer cell lines, including SKOV3 ovarian, T24 bladder, MDA-MB-231 TNBC, PC3 prostate, and A549 lung tumor cell lines. Surface TROP2 expression is expressed as mean fluorescence intensity.
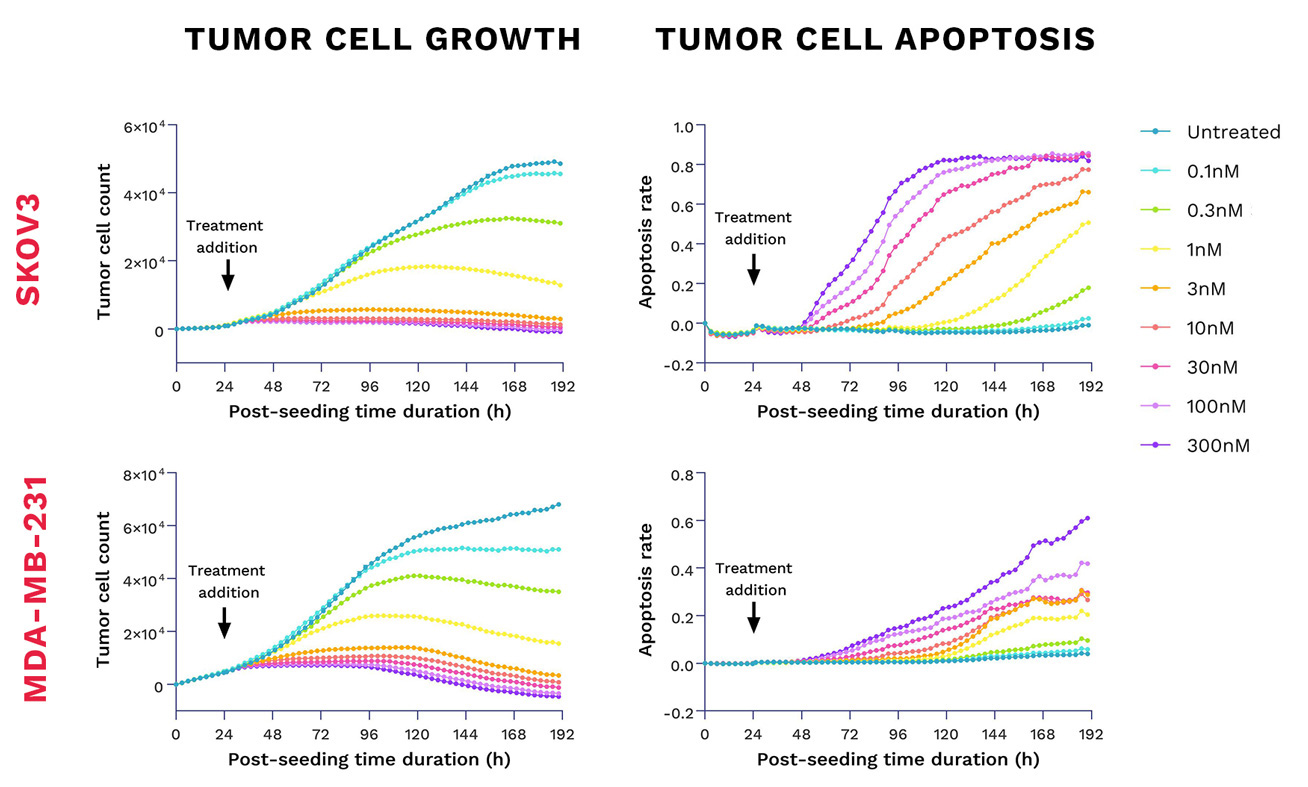
TROP2-ADC exhibits differential tumor anti-proliferative and cytotoxic activity against TROP2-positive cell lines
TROP2-ADC – Sacituzumab govitecan – displays potent cytotoxicity against the TROP2-positive SKOV3 and MDA-MB-231 tumor cell lines. Cells were seeded at their appropriate working densities, and treated with the TROP2-ADC for dose-response function. Tumor cell growth and apoptosis were evaluated kinetically by live cell imaging over a 7-day period post-treatment. All the readings were normalized to the baseline.
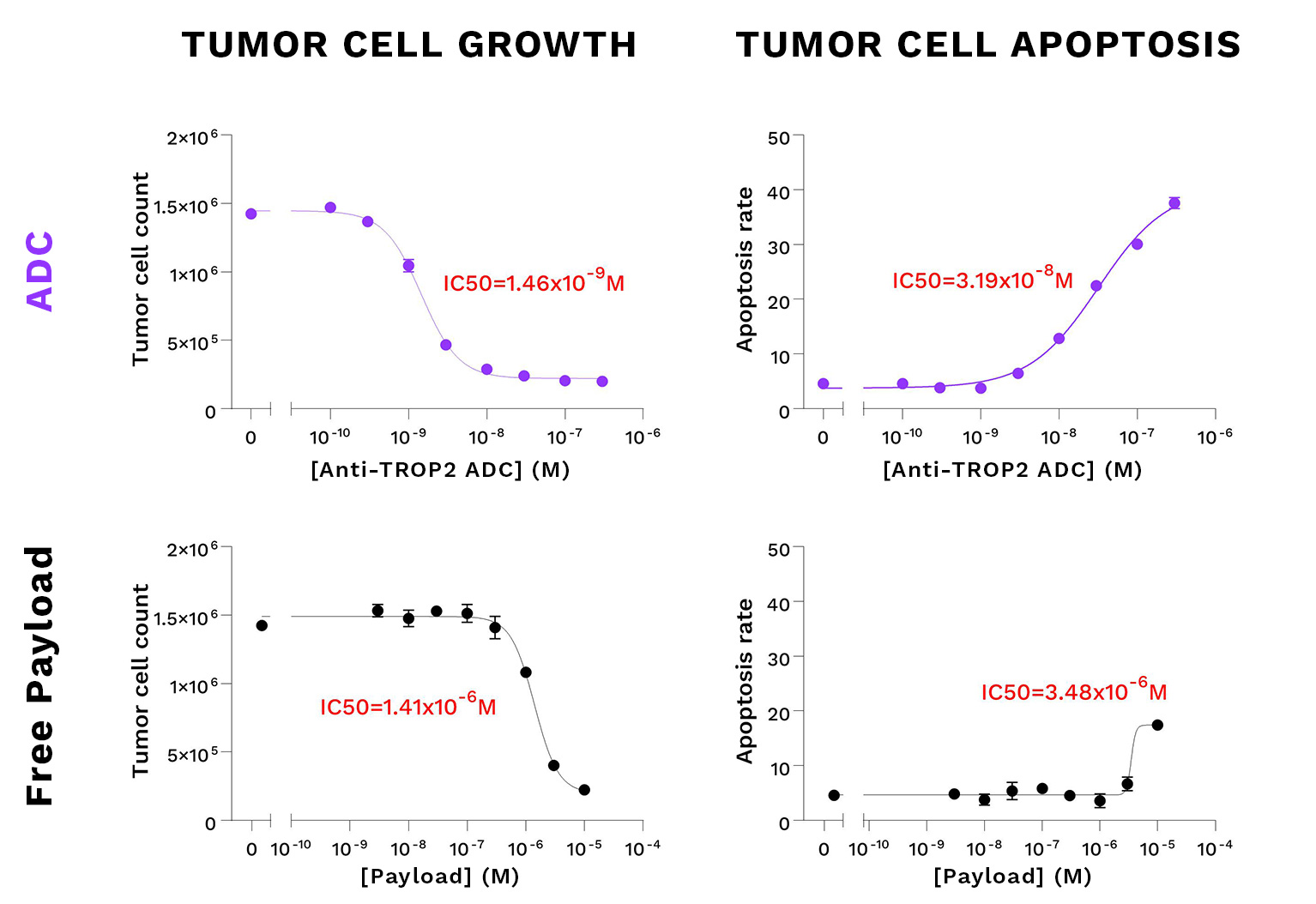
Growth inhibition & cytotoxicity on SKOV3 tumor cells: ADC vs payload
TROP2-ADC – Sacituzumab govitecan – remarkably reduces the cell growth and induces apoptosis of SKOV3 cells, compared to Irinotecan, the prodrug of SN38 (the ADC drug conjugate). Cells were seeded at their appropriate working densities, and treated with either the TROP2-ADC or the free payload for dose-response function. Tumor cell growth and apoptosis were evaluated kinetically by live cell imaging 4 days post-treatment and represented as AUC over this duration.
Why working with Explicyte?
Experts
in Immuno-Oncology
- 100+ in vitro campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- A comprehensive technology platform to validate targets, and assess the efficacy, mechanism of action and toxicity of novel compounds
Personalized
approach
- Targeted discussion based on your request to design bespoke strategy & fit-for-purpose study proposal
- A dedicated study director (PhD level) from experimental plan to final report discussion
- Custom cellular models: over 100 human cancer cell lines available, cultured in 2D or 3D.
Tell us about your project !

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)
Antibody Drug Conjugates (ADC) discovery & proof-of-concept services I in vitro CRO services
Explicyte supports ADC developper in the analysis of their target(s) expression in human specimens and cell lines and provided in vitro services for the analysis of antibody-drug conjugate binding, specificity and internalization, as well as in vitro tumor killing assays to assess ADC efficacy. Our CRO services also allow to assess ADC off-target effects and ADC via in vivo and in vitro investigations characterizing ADC immunogenicity and tissue cross-reactivity.










