-
- Model features: CD8+ T cell-mediated control of tumor growth, MDSCs tumor infiltration
- Tumor cell line: CT26 tumor cells
- Tumor implantation: subcutaneous
- Standards: immune checkpoint inhibitors (anti-PD1, PDL1, CTLA4, 5FU)
- Readouts: body weight, tumor size, survival
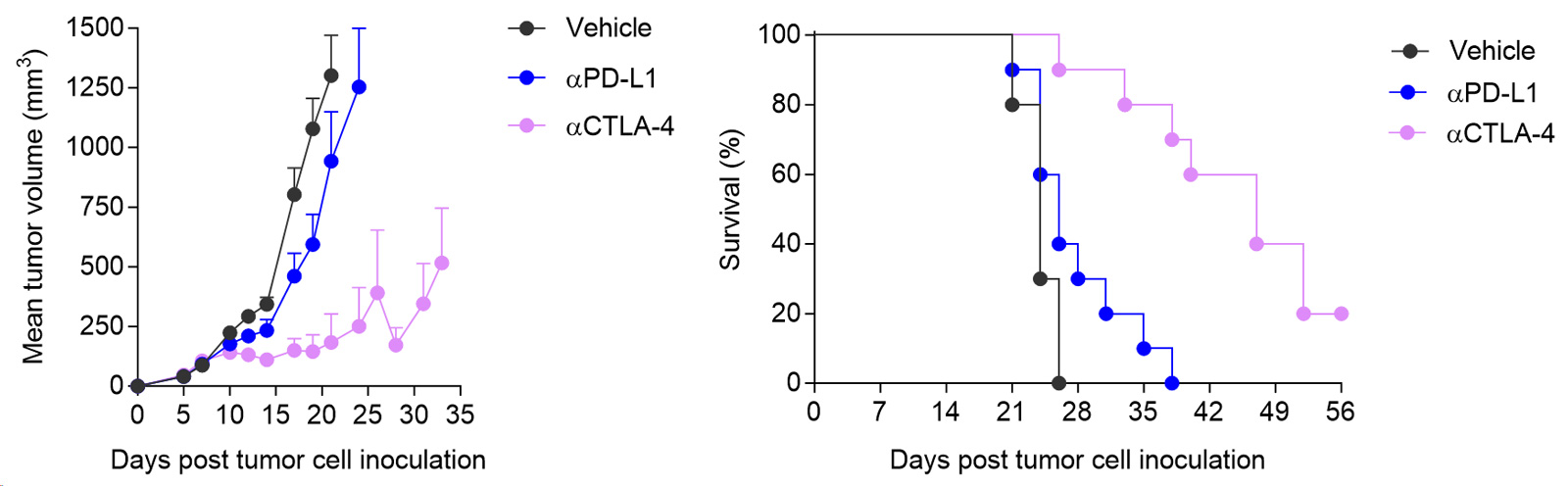
Subcutaneous CT26 colon tumor model is differentially sensitive to immune checkpoint blockers
Mice were challenged with CT26 cells and treated with either isotype, anti-PDL1 or anti-CTLA4 antibodies. Tumor growth and survival were monitored overtime.
In vivo efficacy & mechanism of action studies for novel immunotherapies
Straightforward in vivo efficacy studies
- N=10: Standard groups of 10 mice including groups exposed to test compound alone and in combination with reference therapy.
- Weekly reports: monitoring tumor growth, body weight, and survival
Flexible sampling options
- Monitoring response over time: satellite mice, serial bleeding, intra-tumoral biopsies
- On-demand sample collection: blood, serum, plasma, tumor, organ samples
A flexible platform to quantify tumor-microenvironment & peripheral markers
- Multiplex immunophenotyping by flow cytometry & digital pathology
- Spatial transcriptomics & proteomics
- Tumor microdialysis
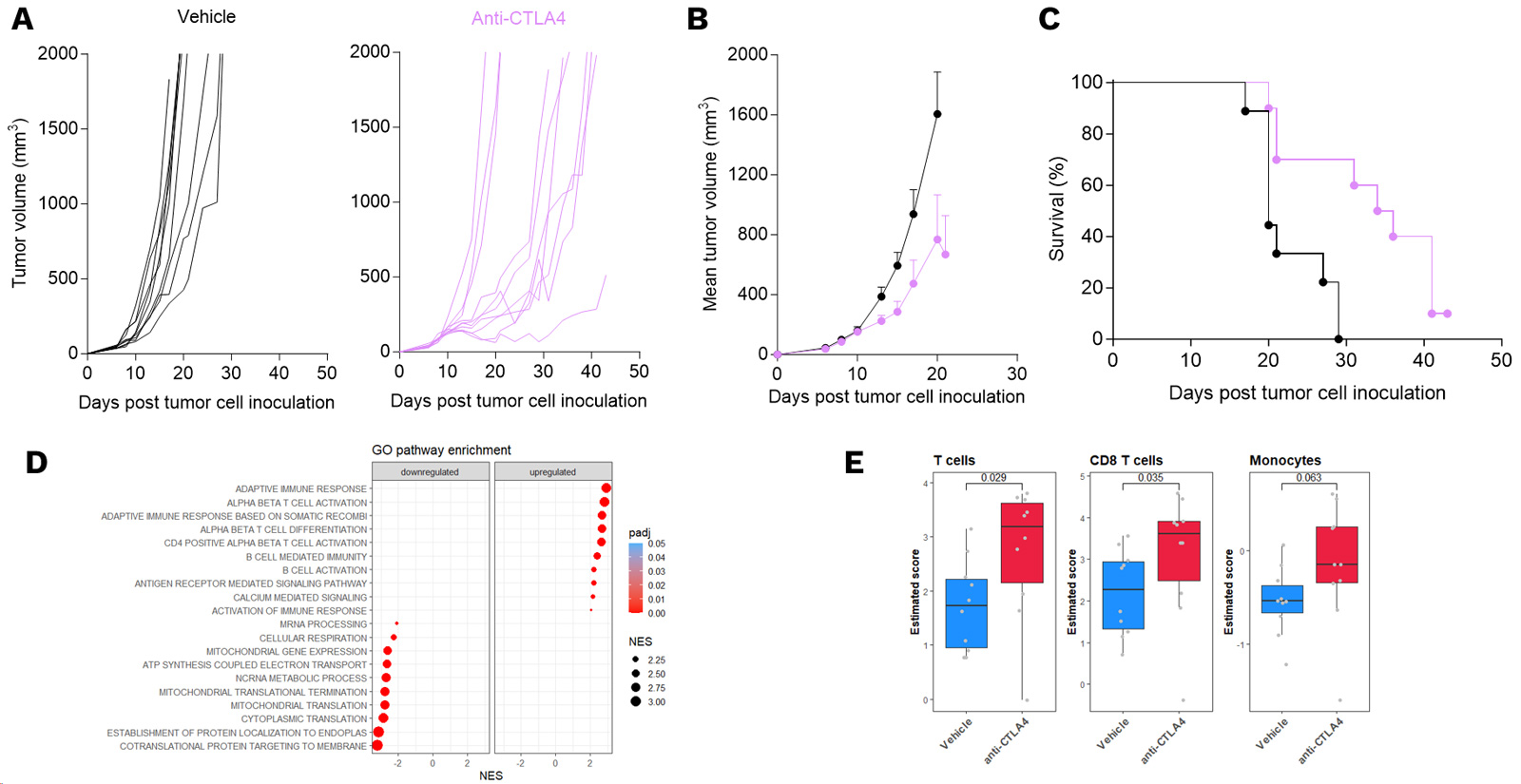
Characterization of the mechanism of action underlying the anti-tumor activity of anti-CTLA4 in a mouse model of CT26 colon carcinoma.
Mice were subcutaneously inoculated with CT26 tumor cells and treated with anti-CTLA4. Tumor growth and survival were monitored over time. Individual tumor growth (A), mean tumor volume (B), and survival (C) are presented. During treatment, tumor biopsies were collected and processed for RNA sequencing and analysis of GO Pathway (D) and immune cell (E) enrichment upon anti-CTLA4 exposure, both highlighting strong immune system activation.
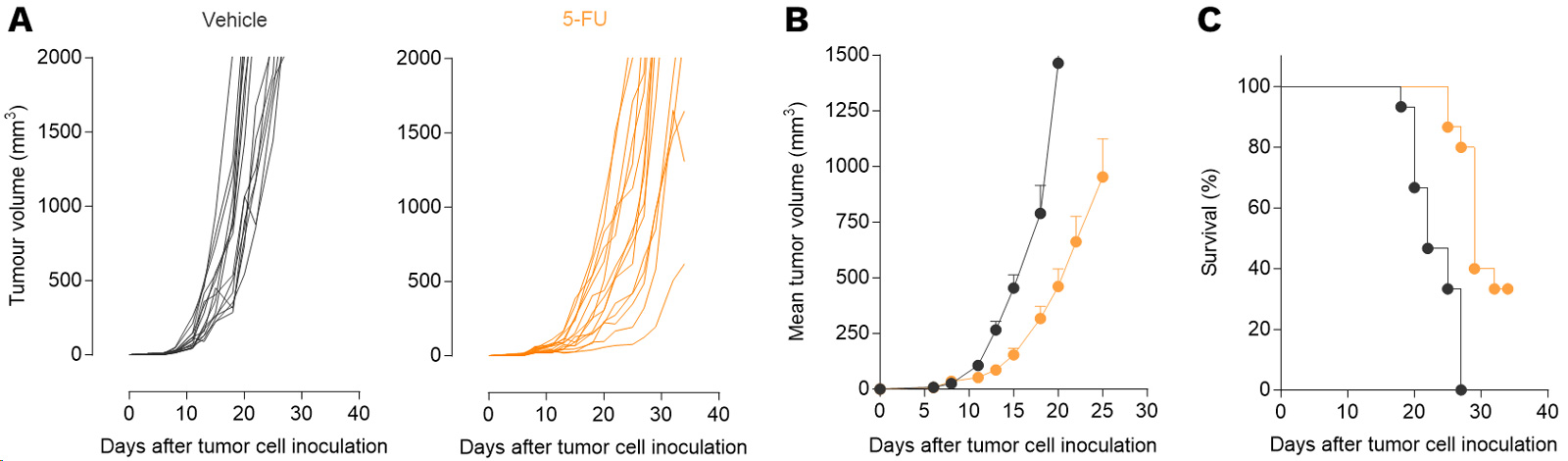
5FU exerts anti-tumor activity in a CT26 mouse colon cancer model.
Mice were subcutaneously inoculated with CT26 tumor cells and treated three times with 5FU. Tumor growth and survival were monitored over time. Individual tumor growth (A), mean tumor volume (B), and survival (C) are presented
Why working with Explicyte?
Experts
in Immuno-Oncology
- 150+ in vivo campaigns conducted over the past 10 years
- 30+ peer-reviewed publications in key immuno-oncology journals
- Bespoke study designs based on client objectives and literature
Personalized
approach
- A dedicated study director (PhD level) from experimental plan to final report
- Weekly reports to provide regular updates & adapt experimental strategy
- Comprehensive analytical platform to decipher anti-tumor response
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)