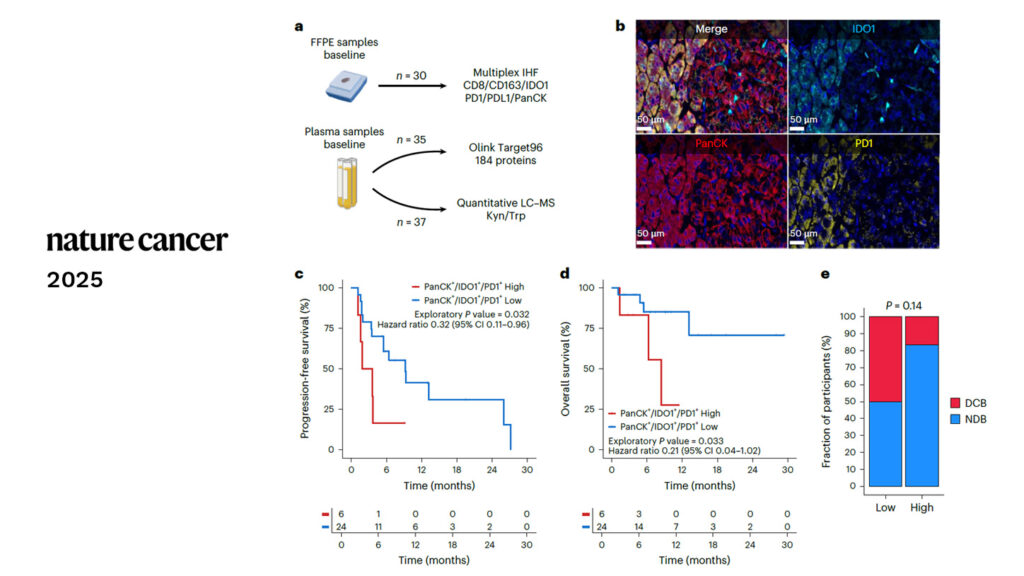
Over the past decade, Explicyte has partnered with pharmaceutical companies and Comprehensive Cancer Centers in the context of translational biomarker programs and early-phase clinical trials.
These collaborations led to the publication of 30+ peer-reviewed papers in top-tier journals, characterizing the response/resistance to cancer immunotherapies and identifying novel predictive biomarkers of anti-tumor immune response.