Since 2019, Explicyte has leveraged archival FFPE tumor tissues for target and biomarker discovery, starting with NanoString GeoMx. Building on 30+ peer-reviewed publications, we expanded in 2024–2025 with the full 10x Genomics suite (Chromium X, Visium HD, Xenium) and became a certified service provider—enabling fit-for-purpose FFPE transcriptomics from study design to decision-ready target shortlists.

Explicyte operates a comprehensive platform to support oncology target discovery campaigns in FFPE cohorts, from sample strategy to decision-ready outputs:
- Custom FFPE cohort design & biospecimen sourcing: fit-for-purpose cohorts aligned to your indication, subtypes, and clinical variables, with associated metadata.
- FFPE QC & controlled processing: pathology review and pre-analytical checks to de-risk studies early.
- Strategic technology selection: guidance to choose the right approach based on gene coverage (whole-transcriptome vs focused panels), resolution (single-cell vs ROI/region-based), and throughput.
- In-house bioinformatics & biostatistics: robust workflows for dataset remapping (when needed), QC, cleaning, and annotation, plus custom analyses integrating clinical metadata (stratification, differential expression, signature scoring, and decision-support statistics).
On-site capacity: Xenium x2, Visium HD x1, Chromium X x1, GeoMx x1 (FFPE-ready workflows).
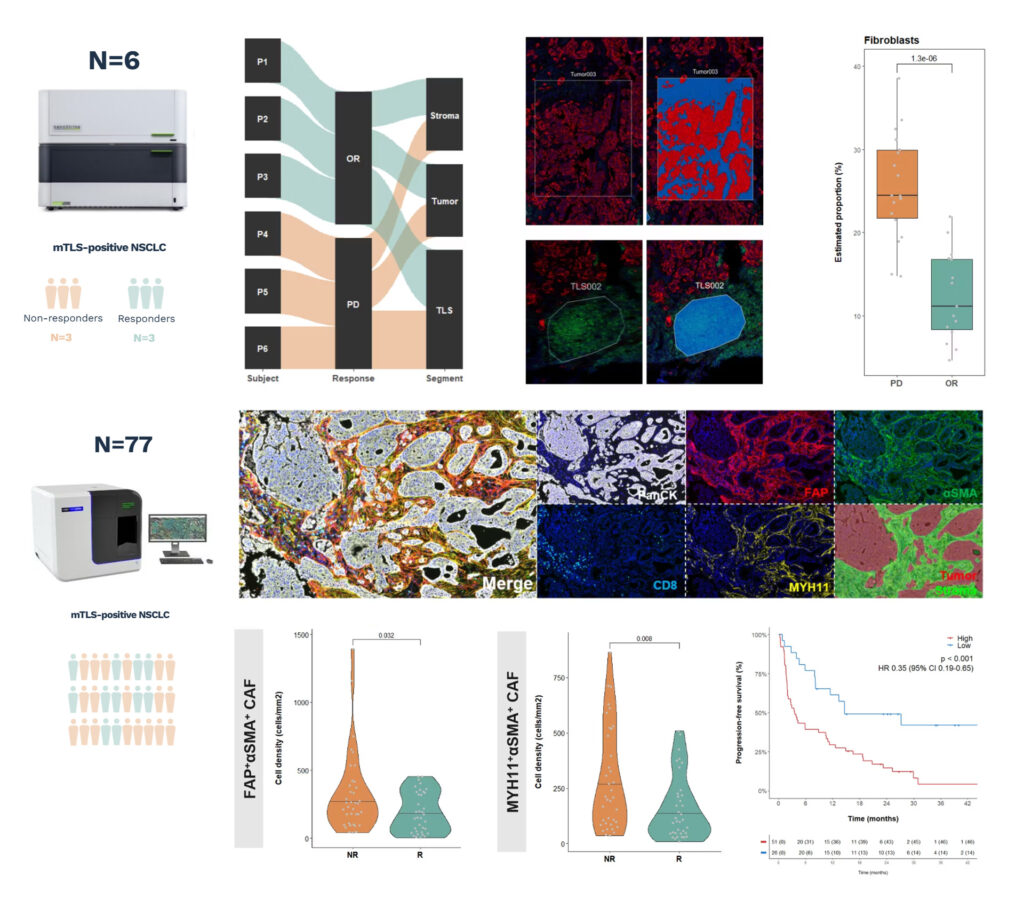
1) Identify targets in a small number of extreme cases using transcriptomics, validate target expression in a larger cohort by digital pathology
We employed this strategy in a 2025 paper in Cell Reports Medicine: GeoMx was used in 6 extreme profiles (responder vs non responders) to identify determinants of resistance to immunotherapy. This hypothesis was then validated using a multiplex IF panel in 77 cases.
- The question: Why do some patients with NSCLC, despite featuring mature tumor lymphoid structures (mTLS) — a typically favorable phenotype — still fail to respond to anti-PD-1/PD-L1 immunotherapy?
- Spatial transcriptomics (GeoMx insights): Within NSCLC patients with mTLS, non-responders feature an enrichment in fibroblasts in the stromal compartment
- Digital pathology validation: Non-responders feature higher stromal density of FAP+αSMA+ CAF and MYH11+αSMA+ CAFs. Presence of CAF subsets correlates with poor outcome.
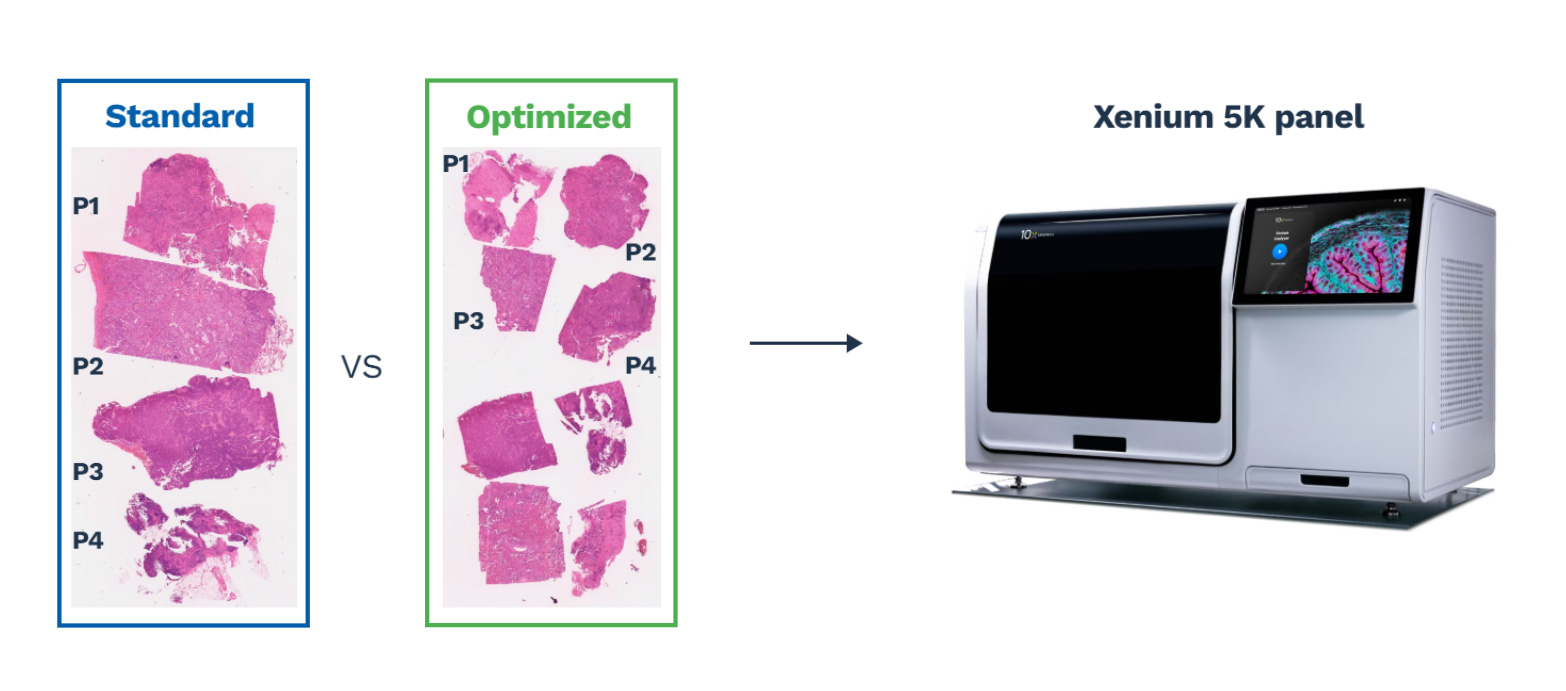
2) Sample multiplexing (Xenium optimization)
To cut turnaround time and cost on the Xenium, we recently validated we could double samples per slide without compromising data quality. We demonstrated that:
- Sample downsampling preserves sample quality and output
- Tissue heterogeneity is preserved with downsampling, with stable cell-type proportions
- Gene expression is maintained with downsampling
3) Efficient data science workflows to deliver actionable datasets faster
Through internal R&D, our data science team established workflows to process & analyze high-dimensional datasets efficiently:
- Validated transcriptomics dataset processing for fast data remapping, cleaning, and annotation
- Target prioritization strategies taking into account your envisioned modality
- Custom dataset analysis to support decision-making (custom spatial analysis, differential gene expression plots, gene signature & pathway analysis, in-depth statistics with clinical metadata)
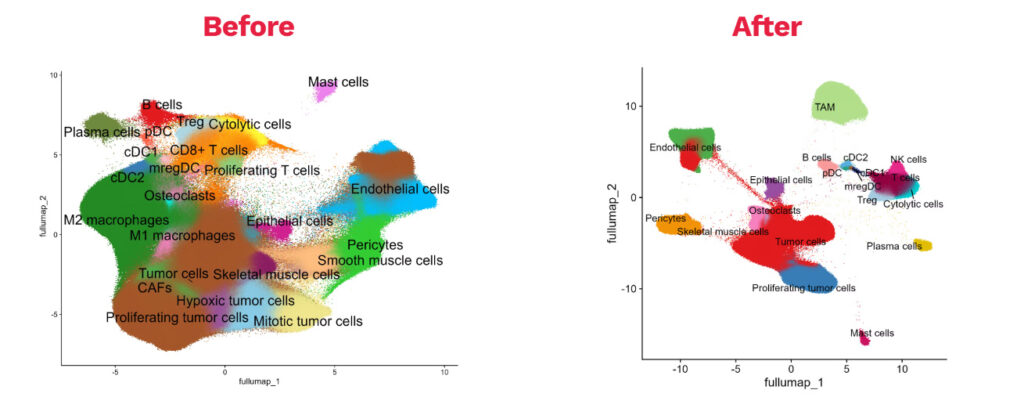
Illustration of our data processing workflow on Xenium dataset: raw vs pre-processed data
Experts
in Immuno-Oncology
- Certified service provider for 10x Genomics FFPE transcriptomics workflows
- Multi-platform capacity (Chromium X, Visium HD, Xenium x2, GeoMx) to match question, budget, and throughput
- Track record of 30+ peer-reviewed publications leveraging spatial/transcriptomic data in clinically relevant settings
Personalized
approach
- PhD-level study director providing strategic advice and regular updates
- In-house processing pipelines (remapping when needed, QC, cleaning, annotation) + custom analyses with clinical metadata
- IP-friendly collaboration: we do not claim ownership of sponsor-derived targets; project outputs are delivered under your contract terms.
Your contacts

Talk to our team !
Paul Marteau, PharmD (preclinical study director), Imane Nafia, PhD (CSO), Loïc Cerf, MSc (COO), Alban Bessede, PhD (founder, CEO), Jean-Philippe Guégan, PhD (CTO)















