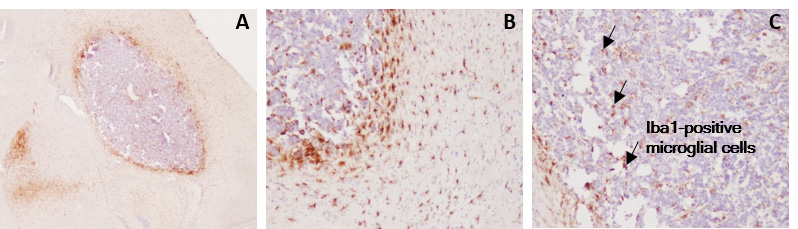
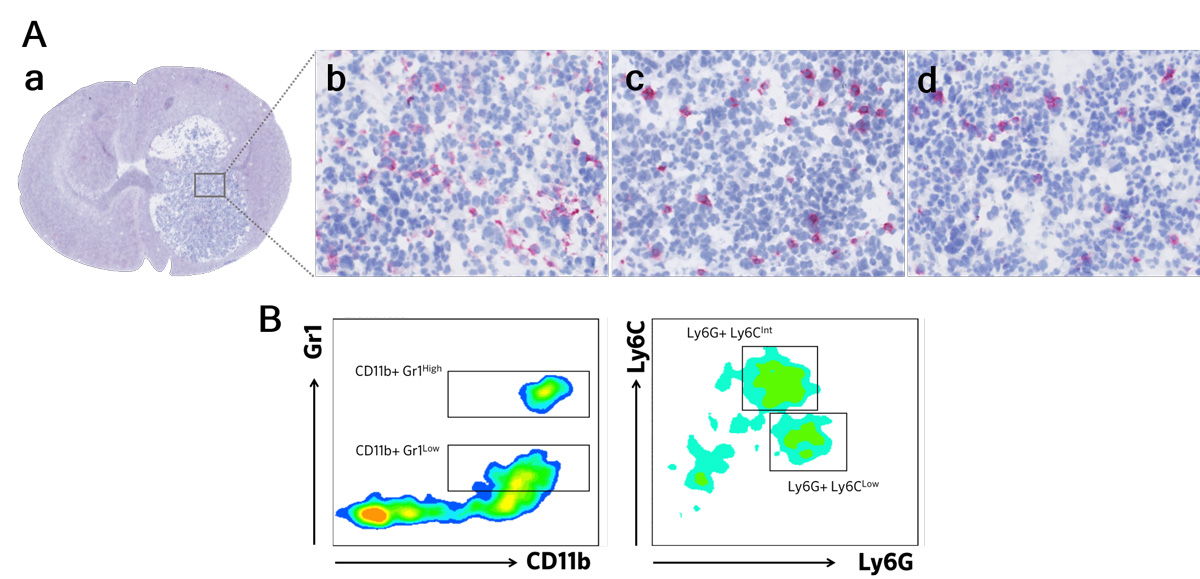
In vivo orthotopic glioblastoma model
Apart from the advantage of the tumor cells (stably expressing the 2nd generation firefly luciferase) which are intracranially implanted by stereotactic surgery, our syngeneic GBM model mimics the major human disease features including immune cell activation and infiltration, and astrogliosis, among other characteristics.
Moreover, our GBM model was set up and validated to assess novel combination regimens with chemotherapy or immunotherapy. Robust treatment protocols combined with validated gold standard chemotherapeutics and immune checkpoint inhibitors have been optimized and allowed to characterize and validate GL261 glioma as responsive to Temozolomide (TMZ) and to anti-CTLA-4 treatment.