Immunosuppressive Tumor Microenvironment In OT 4T1 Breast Tumor Model
10 / 16 / 2017
While known and validated as non-responsive to conventional immune checkpoint inhibitors, our OT syngeneic mammary tumor model, which represents an aggressive model of human breast carcinoma, is characterized by the presence of at least two types of tumor-infiltrating immunosuppressive cells – myeloid suppressive cells (MSC) and T regulatory cells (Treg). Such cell populations are known, as a result, to suppress effector immune cell responses, thereby promoting tumor immune escape and metastasis.
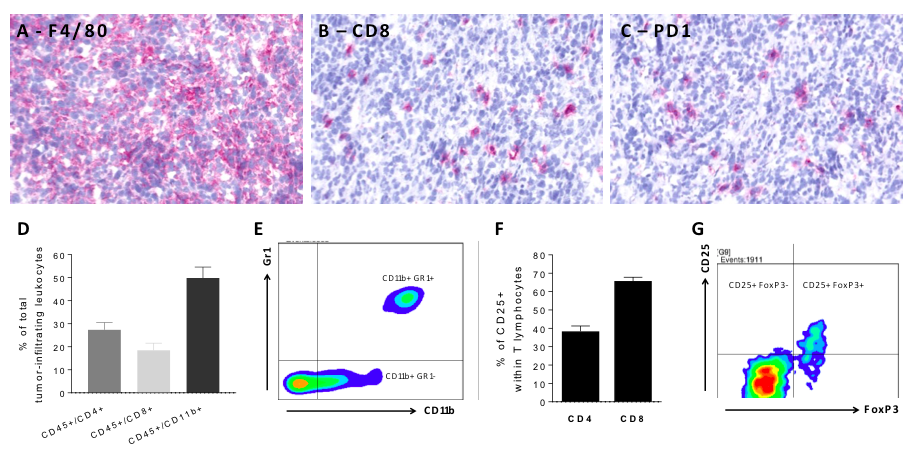
Presence of myeloid and Treg tumor-infiltrating immunosuppressive cells in orthotopic 4T1 breast tumor-bearing model. IHC staining of 4T1 tumors shows the presence of F4/80+ macrophages (A) and of CD8+ cytotoxic lymphocytes infiltrating the tumor (B). Presence of tumor-infiltrating PD1-expressing immune cells is highlighted in(C). (D) Flow cytometry analysis of 4T1 tumors shows infiltration by CD4 and CD8 lymphocytes, and by myeloid CD11b+ cells. (E) Myeloid CD11b+ are further analyzed as Gr1- or Gr1+. (F) Flow cytometry analysis also shows the presence of activated CD25+ CD4+ and CD8+ lymphocytes. (G) Activated CD4 lymphocytes are further analyzed for FoxP3 expression to identify Treg cell population.